From (by) RocketSlinger@SBCGlobal.net (email me there please)… This is a sub-site
to main site at www.rocketslinger.com …
This web page last updated 11 November 2023
A Classy,
Sassy, and Brassy Glass-Class on LionGlass, RoboGlass, and ArtGlass
Abstract
This sub-page to www.ResearchGate.net (https://www.researchgate.net/publication/375576535_A_Classy_Sassy_and_Brassy_Glass-Class_on_LionGlass_RoboGlass_and_ArtGlass#pf1c
) is duplicated at www.rocketslinger.com also (at http://www.rocketslinger.com/LionGlass/ ). This
is purely speculation and internet research here. No experimental glass materials were produced
or tested, by the author of this paper.
Links to articles and research papers are provided, for LionGlass (from
Penn State University), and other materials that might be combined with
LionGlass. Such materials include other
types of glasses, Kovar, perlite, pumice, borax, boric acid. “water glass”, calcium
bicarbonate, hydrates of sodium carbonate, silica gel, glass microspheres and micro-balloons, glass tubes,
syntactic foams, foam glass, ceramic paper, many types of “grit” and fiber, and
many more. Techniques discussed here
include the float-glass method, slumping, fusing, and exotic (proposed) new
methods. A proposed new tool named a
“hot-glass gun” (evoking a molten-plastics-dispensing hot-melt glue-gun, but,
of course, much hotter) is described here.
Potential applications here include structural and
artistic products such as wall and floor tiles, window panes, kitchen counters,
construction blocks, vessels (mugs, cups, bottles, jars, etc.), and COPV-like
pressure vessels.
Contents
Preamble, and Bits of Boilerplate
A Palette of
Glass-Associated Materials.
Materials
Behaviors of Glass (and Ceramics)
Possible New
Tools for Glass-Working
Applying Hot
Glass to Hot Glassware
COPV-Like
Glass Pressure Vessels
Example Art-Glass
Compositions
Preamble, and Bits of Boilerplate
As before, as with
other sub-pages of www.rocketslinger.com, the intent here is to “defensively publish” miscellaneous ideas,
to make them available to everyone “for free” (sometimes called “throwing it
into the public domain”), and to prevent “patent trolling” of (mostly) simple,
basic ideas. Accordingly, currently-highly-implausible or
speculative design ideas (frequently marked as such) are sometimes included,
just in case they ever become plausible, sometimes through radical new
technology developments (often in materials sciences).
Let me add, I have no problems at
all with well-deserved patents, such as the patent(s) for “LionGlass” currently
being applied for, by the Penn State researchers. They are a team led by Dr. John Mauro,
FYI.
It is the obvious and overly-broad patents that bother me. For a humorous take on all of this, see http://www.rocketslinger.com/Near_Universal_Defensive_Publication/ . For a recent
POSSIBLE example (I haven’t read the patent, so I’m trying to not be too
judgmental), see https://www.slashgear.com/1092749/why-nuclear-fusion-could-be-the-key-to-air-superiority/ “Why Nuclear Fusion Could Be The Key To Air Superiority”.
From there, “Lockheed
Martin filed a patent for a fusion-powered jet several years ago.” Patenting uses for controlled thermonuclear
fusion, years or perhaps decades before such things become practical and
affordable? Well, if they can do that, I
can “defensively publish” speculative uses for LionGlass etc., for sure!
Dear Reader,
excuse me as I will often slip out of stilted formal modes of writing
here. I have no boss or bosses to please
with these “hobby” writings of mine, so I’ll do it my way! I’ll
often use a more informal style from here on in, using “I”, “we”, “you”,
etc. “We” is you and me. “You” are an engineer, manager, or other
party interested in what’s described here.
Let’s thwart the patent trolls, and get ON with it!
More-Specific Introduction
First
off, in the “digression” or “random misc.” category, I have lately been in the
habit of adding corrections or comments concerning my most resent paper, in my
newest paper. I’ve not found a way to
update older papers already posted on “ResearchGate”. My most recent paper here has been https://www.researchgate.net/publication/369143849_Aerostat_Maneuvering_Techniques_and_Hiding_Rockets_from_Zombie_Radar . A lot of that paper was about hiding from
radar by using low-dielectric-constant vehicle-construction materials. I had my sneaking suspicions that this (as
opposed to using RAM, Radar-Absorptive Materials) was a waste of time. Now I have confirmation that this is (probably,
largely) true. Modern radars can see
VERY well! See https://news.yahoo.com/general-atomics-radar-turn-gray-181215950.html
“General Atomics: New radar to turn Gray Eagles
into anti-drone hunters” From there…
“Eagle Eye was able to detect and track a small
fixed-wing drone made out of balsa wood — much smaller than forces in the field
would likely encounter from an enemy.” So much for
radar-invisible construction materials (such as wood), then… End of this particular digression!
In
the mode of at least SLIGHT digressions, let me also note that some of my past
papers were (perhaps in the minds of some readers) cluttered up with a LOT of
links to some fairly easy-to-find (easy-to-“Google”) information. https://www.researchgate.net/publication/369143849_Aerostat_Maneuvering_Techniques_and_Hiding_Rockets_from_Zombie_Radar for example
had lots of links-sources for dielectric constants of many types of
materials. https://www.researchgate.net/publication/365964188_Propulsion_Designs_Using_Novel_Nested_COPVs_and_the (incomplete
title in the link, but there it is) shows MANY handy links (sites) for units
conversions, and many types of materials parameters (including costs) for many
types of materials. So of course, if
you’re interested in such things, check out these papers!.
This time around, I’ll try not to
clutter up this paper with easy-to-find facts.
Also excuse me as I sometimes will repeat my favorite (most relevant)
links several times, in different contexts.
At the ends of certain sections, I may add a bit of clutter with “dumps”
of less-than-stellar links that MIGHT be of interest, too. If I don’t include ENOUGH links and sources,
and you need some help in that category (or other categories), please email me
at RocketSlinger@SBCGlobal.net.
Onward,
now, to the actual introduction… Here,
we’ll first review a “palette” of useful materials, starting with LionGlass,
and moving towards less-and-less plausible-for-common-uses (more expensive,
more exotic) materials. I will sneak in
“previews of coming attractions” (techniques, tools, and applications) as we go
through materials. Then we’ll cover
techniques, and then tools, most especially to include a “hot-glass dispenser
tool” or “hot-glass gun”, as envisioned by Yours Truly. Then we’ll describe potential applications in
a fair amount of detail. Repeating from
the above abstract, potential applications
here include structural and artistic products such as wall and floor tiles,
window panes, kitchen counters, construction blocks, vessels (mugs, cups,
bottles, jars, etc.), COPV-like pressure vessels, and telescope mirrors.
A Palette of Glass-Associated Materials
“LionGlass” is the lead character in this show, clearly! I couldn’t find detailed specifications (yet)
for costs, density, CTE (coefficient of thermal expansion), mechanical
strengths (in compression or tension modes), or certain other
materials-behaviors facts or parameters.
As we can see from the below links, LionGlass’s melting point is
significantly lower than other glasses, and far less fracture-prone, when
tested at 10 or more times more force, compared to older types of glass (when
poked with a sharp point, presumably a sharp point of diamond, as in the
Vickers test; see https://en.wikipedia.org/wiki/Vickers_hardness_test ). Importantly, I do
not know LionGlass’s (quite important) thermal expansion and contraction
coefficient (CTE, https://matmatch.com/learn/property/what-is-coefficient-of-thermal-expansion ). The best link (with the
most details) that I could find, concerning LionGlass, is here: https://www.glass-international.com/news/lionglass-a-low-carbon-step-towards-a-sustainable-future#:~:text=Soda%20lime%20silicates%20have%20a,of%201400%2D1500%C2%B0C.&text=Considering%20that%20carbon%2Dbased%20energy,carbon%20footprint%20of%20its%20own Quoting from there, ” A molten glass that reaches a target viscosity at a lower
temperature is safer and more affordable to work with. For example, soda lime
silicate reaches its softening point, the point where it begins to slump
underneath its own weight, at about 730°C.8 LionGlass reaches this same point
more than 100 degrees lower, at about 590°C.”
From this immediately-above-linked
source, see “Figure 1”, for viscosity (liquid “thickness”) v/s temperature for
soda-lime glass and for LionGlass. To
understand that “molten” glass can be VERY viscous (thick), think of
glassblowers that you’re seen in action,
or videos thereof (or go and look one up).
For a small look-ahead “cheat”, please consider that I
would like to see what happens if un-expanded perlite expands in LionGlass. This (perlite expansion) happens at 1,600 degrees F (871 degrees C). 871 C fits in a VERY nice spot in the
above-referenced viscosity v/s temperature graph for
LionGlass. Such a blend of expanded
perlite and LionGlass should be less dense (heavy) than LionGlass, and probably
more affordable, and may offer other benefits, too.
To put LionGlass into context, not
only with respect to soda-lime glass, but to other types of glass as well, for viscosity
v/s temperature, see https://glassproperties.com/viscosity/ … This one excludes
the newer “LionGlass”, but does show 100% silica glass (AKA quartz glass),
which has a nearly over-the-top (very high) melting point. It is, however, also over-the-top expensive,
generally speaking, sad to say.
A grab-bag of links that I have
gathered concerning LionGlass (less useful to me at least, than my favorite
such link, as above) follows: https://scitechdaily.com/lionglass-new-type-of-glass-thats-greener-and-10x-more-damage-resistant/ and https://news.yahoo.com/researchers-develop-game-changing-glass-210000236.html and https://www.psu.edu/news/research/story/new-glass-cuts-carbon-footprint-nearly-half-and-10x-more-damage-resistant/ and https://www.psu.edu/news/research/story/new-glass-future-taking-lionglass-out-lab-and-market/ . Also https://happyvalleyindustry.com/penn-state-was-a-clear-choice-for-gorilla-glass-inventor-no-pun-intended/ .OK, one more, it’s pretty
good: https://www.glass-international.com/news/lionglass-a-low-carbon-step-towards-a-sustainable-future#:~:text=LionGlass%20was%20created%20to%20have,C%20to%201500%C2%B0C . … Enough yet? If not… Go Google!
Finally, let me add that I realize
that LionGlass (like its predecessor, Gorilla Glass) will most likely be too
expensive to be used in large quantities, for glass-art purposes. In that case, feel free, when reading all of
the below, to take “LionGlass”, and substitute for it, “LionGlass or any other
suitable future material, especially a more affordable material”.
“Perlite” can sometimes be called “mineral microspheres” (especially, I suppose, in its
expanded form). See https://ogma.newcastle.edu.au/vital/access/manager/Repository/uon:28964#:~:text=Perlite%20is%20a%20glassy%20volcanic,resistance%20and%20water%20retention%20properties. “Elastic
and mechanical properties of expanded perlite and perlite/epoxy foams” From
there, “Perlite is a glassy volcanic rock of silicic
composition and in its expanded form has a high porosity (>95%), low density
(~ 0.18 g/cm³) and offers excellent thermal and acoustical insulating
properties, chemical inertness, physical resilience, fire resistance and water
retention properties.” Also from there, “Syntactic foams have superior mechanical and thermal
properties but they are more expensive and denser than conventionally gas-blown
foams.” Note that “syntactic foam”
usually refers to a matrix (bonding agent) of epoxy, but I would like to see
someone attempt to use LionGlass (or other glass) instead of epoxy, for
that. We’ll look at “syntactic foam”
more later… If
not-much so in this paper, perhaps in a future paper!
See https://mineralseducationcoalition.org/minerals-database/perlite/#:~:text=Perlite%20is%20an%20amorphous%20volcanic,twenty%20times%20its%20original%20volume . From there, “Perlite is an amorphous
(with no defined crystalline form) volcanic glass (silica or SiO2) that
has relatively high water content, typically formed by the hydration of
obsidian. Perlite
has the unusual characteristic of expanding and becoming porous when it is
heated. It can expand to as much as
twenty times its original volume.
Expansion occurs when the glassy lava rock is
heated to 1600 degrees F (871 degrees C) and the water molecules trapped in the
rock turn into vapor, causing the rock to expand.” Note the 871 degrees C, which would work
nicely with blending the expanding perlite with molten LionGlass (and possibly
other ingredients as well, in “TBD” ratios), to experimentally determine what
useful material blends might result.
What is the difference between obsidian
and perlite? And pumice? See https://www.alexstrekeisen.it/english/vulc/glass.php#:~:text=Obsidian%3A%20is%20usually%20a%20black,less%20than%200.5%20%25%20of%20water.&text=Perlite%3A%20is%20a%20gray%20or,result%20from%20hydration%20of%20obsidian. From above, “Obsidian:
is usually a black, massive glass with a shiny, vitreous luster and conchoidal
fracture. Is a rhyolitic glass with less than 0.5 % of water.
“Perlite:
is a gray or pale brown glass, very brittle and highly fractured, with
spherical cracks. Perlite glass probably result from hydration of obsidian.
“Pumice:
is a highly vesiculated, cellular glass froth.”
Also from above, “ Essentially all glass older than Mesozoic has been
devitrified.”
FYI, glass is “amorphous”. “Devitrified” means it has “degraded” to a
crystalline or containing-crystals form.
OK, “degraded” may be too judgmental…
Which is “better”? Crystals, or glass?
It depends on what you want to do with it! I want to concentrate on “glass” here,
mostly.
We’ll address pumice
soon. It is nature’s version of
human-made “glass foam”, both of which will look into some more, further below. So anyway, perlite and LionGlass blends… What would happen? I for one would sure like to know! Now here is your grab-bag of more associated
links: https://www.researchgate.net/publication/366833239_Modelling_the_Thermal_Treatment_and_Expansion_of_Mineral_Microspheres_Perlite_in_Electric_Furnace_Through_Computational_Fluid_Dynamics_CFD_Effect_of_Process_Conditions_and_Feed_Characteristics “Modelling the Thermal
Treatment and Expansion of Mineral Microspheres (Perlite) in Electric Furnace
Through Computational Fluid Dynamics (CFD): Effect of Process Conditions and
Feed Characteristics” and https://www.researchgate.net/publication/304981769_Novel_Cellular_perlite-epoxy_foams_effects_of_particle_size “Novel Cellular perlite-epoxy foams: effects of
particle size” and https://www.researchgate.net/publication/303832199_Novel_cellular_perlite-epoxy_foams_Effect_of_density_on_mechanical_properties “Novel cellular perlite-epoxy foams: Effect of
density on mechanical properties”.
“Pumice” is another possible ingredient to add to, or blend with,
LionGlass. It has already been mentioned
above, as we explored “perlite”. Pumice
could, for example, be sprinkled on top of cooling-down glass panes, to create
floor tiles. Pumice is “gritty” and
would add friction (roughness) to this surface, to prevent people (and their
pets!) from “slip-sliding away”! I’ve
not gathered many links about pumice, but you can “google” it as well as I can,
no doubt.
For the melting point of
pumice, see https://link.springer.com/article/10.1007/BF02597176#:~:text=Thermal%20experiment%20on%20the%20pumice,occurs%20at%201350%C2%B0%20C. “Experimental study on pumice and obsidian” From there, “Thermal experiment on
the pumice at atmospheric pressure shows that welding begins at about 900° C
and complete melting occurs at 1350” It
sounds like it could be used for a surface treatment for LionGlass! Or for a surface treatment of perlite expanded
into LionGlass at 871 C!
That’s all that I have for pumice. “Google” away for more, and-or consult an
AI! I am sure that an AI can
“hallucinate” answers here for you, as well as the rest of us can!
“Other
Minerals and Types of Sand” are other
possible low-cost ingredients here (to be blended with LionGlass), as well as
other types of glass, or glass crushed into sand particles. Obsidian glass and other natural forms of
glass come to mind. For more types of
glass, ask The Google! Soda-lime glass, borosilicate glass, quartz glass, lead glass, and
more. See https://www.cmog.org/article/types-glass . Barium titanate
glass is yet another type of glass; see https://www.cospheric.com/barium_titanate_solid_glass_microspheres.htm . Also, types of
glass can be blended. See, for example, https://microspheres.us/materials-for-microspheres/#:~:text=Hollow%20Glass%20Microspheres%20%2F%20Microballoons%20%2F%20Microbubbles,-Microballoons%20and%20Microbubbles&text=Microbubbles%20can%20be%20used%20to,proprietary%20sodalime%2Dborosilicate%20glass%20blend. This source says… “Typically
the glass formulation for hollow microspheres is a proprietary
sodalime-borosilicate glass blend.”
Diatomaceous earth (diatom shells) could possibly serve as an ingredient (to be blended or mixed into LionGlass, for example). “Google” it for yourself! So could many-many types of sand and minerals. I’ll make no attempt to list many more of them, lest I turn this paper into a joke that resembles http://www.rocketslinger.com/Near_Universal_Defensive_Publication/ . The only exception here (an additional type of sand worthy of special mention) is pure-silica or “quartz” sand. This is what (usually) is highly compressed (along with an epoxy binder) to create “engineered stone”, which is a bit expensive, but is often used for kitchen countertops. Perhaps we could substitute LionGlass (for epoxy) as the binder. Thermal expansion coefficients of this sand v/s LionGlass would be HIGHLY relevant here, but I don’t have this number for LionGlass. More about all of this (engineered stone) will be covered later.
“Glass coloring agents” (for stained glass) are other possible (affordable) ingredients here. See https://geology.com/articles/color-in-glass.shtml , or “Google” at will! With LionGlass having a significantly lower melting point than older forms of glass, other coloring agents may become possible (and practical). I do NOT know enough about such things, to speculate usefully, here, so I will quit, now, concerning that. https://en.wikipedia.org/wiki/Glass_coloring_and_color_marking has many notes about metals and metal oxides, and what colors they can impart to what kinds of glass, also. Note that trace amounts of cobalt can create the color blue, here.
https://www.glennklockwood.com/materials-science/glass-compositions.html#glass-additives says “Transition metals'
unfilled d-shells also allow for selective absorptivity when in a glass; for
example, chromia (Cr2O3) gives a green tint
to glass; neodymium (III) oxide (Nd2O3) colors glass purple, and iron (III) oxide (Fe2O3) lends a green tint.” This is an excellent general site concerning
glass, and it discusses other types of glass and glass additives, and infrared
and neutron-radiation absorption, x-ray transmission or absorption, and
refractive indexes, as are associated with different types of glass.
“Kovar” is a metallic alloy whose CTE (Coefficient of Thermal
Expansion) is well matched to conventional types of glass (borosilicate glass
specifically). I don’t know the CTE for
LionGlass, so I don’t know if Kovar is suitable here, or not. I will assume so. If I’m wrong, substitute some other metal or
alloy (I hope that there is one) which matches the CTE of LionGlass, whenever I
mention “Kovar”. See https://en.wikipedia.org/wiki/Kovar for starters. Other types of metals or alloys might be formulated to
match other “COEs” in other glass-types.
“Glass
micro-spheres and micro-balloons” are
other possible ingredients here for mixing into LionGlass. Sad to say, they are NOT very
affordable! They are used in “syntactic
foams”, typically with epoxy as a binder.
One MIGHT be able to replace the epoxy with LionGlass, but I am quite
skeptical about that. Why? See https://microspheres.us/materials-for-microspheres/#:~:text=Hollow%20Glass%20Microspheres%20%2F%20Microballoons%20%2F%20Microbubbles,-Microballoons%20and%20Microbubbles&text=Microbubbles%20can%20be%20used%20to,proprietary%20sodalime%2Dborosilicate%20glass%20blend. “Materials:
What are Microspheres Made of?” Which says, “Typically the glass formulation for hollow microspheres is a
proprietary sodalime-borosilicate glass blend. The thickness of the wall is
most often around 10% of the diameter and the exact density varies dramatically
as a function of the particle diameter of the individual spheres. Because the
shell is so thin, microballoons
are highly fragile and should not be used in high shear processes.” (Emphasis mine.) If we try to blend (mix)
microballoons (even with the balloons being made of higher-melting-point glass
than the LionGlass), the heat will inevitably soften the balloons. Then add the stress of ANY “mixing force”
(stirring force) to the (thick, viscous) mix (even molten LionGlass will be
thick or viscous, resisting mechanical mixing), and these spheres (in my
intuitive mind for sure!) will NOT survive!
I could be wrong! So this
possibility (glass-on-glass syntactic foam) is at least mentioned here, in
passing.
Other than lightening up the density
(weight) of the LionGlass, at fairly high expense, I’m not sure what we’d be
accomplishing here, even if we succeeded, with glass micro-balloons. So I’ll just dump in my few collected maybe-useful
links, and call it quits. See https://iopscience.iop.org/article/10.1088/1757-899X/205/1/012022/pdf#:~:text=In%20contrast%2C%20hollow%20glass%20microspheres,form%20with%20a%20hollow%20structure. “A Comparative Study of
Production of Glass Microspheres by using Thermal Process” Figure #1 there shows a setup for production of these spheres… From there, “In contrast, hollow glass
microspheres are produced by adding a blowing agent to glass powder [3]. Blowing agent such as
sodium silicate decomposes to multiple gases when burned, causing the
microsphere to form with a hollow structure.” Also see https://www.mdpi.com/1996-1944/15/1/81 “Porous SiC and SiC/Cf Ceramic
Microspheres Derived from Polyhydromethylsiloxane by Carbothermal Reduction”
Are there Quartz
glass micro-spheres ?
Yes! See https://santamonicaplastics.com/shop/resins-epoxies-pigments-and-molds/300-quartz-microspheres/ .. But they’re very expensive! And now also don’t forget The Wiki, which,
along with The Google, knows all things!
See https://en.wikipedia.org/wiki/Glass_microsphere , which says “Additional functionalities, such as silane coatings, are commonly added
to the surface of hollow glass microspheres to increase the matrix/microspheres
interfacial strength (the common failure point when stressed in a tensile
manner).” This can serve as a lead-in to
our next topics here…
“Silane and Silane Compounds” are
also of interest, as we now move on to some chemicals that are (or may be)
useful to us here. See https://en.wikipedia.org/wiki/Silanes and https://en.wikipedia.org/wiki/Silane#:~:text=Silane%20(Silicane)%20is%20an%20inorganic,to%20that%20of%20acetic%20acid. Also as previously mentioned right here in
this paper, https://en.wikipedia.org/wiki/Glass_microsphere mentions silane. See also https://www.researchgate.net/publication/273687976_Influence_of_Glass_Fiber_wt_and_Silanization_on_Mechanical_Flexural_Strength_of_Reinforced_Acrylics “Influence
of Glass Fiber wt% and Silanization on Mechanical Flexural Strength of
Reinforced Acrylics”. Phenyltrimethoxysilane is a
specific silane compound likely to be one of the most-likely-use to us here. It has a 131 °C to 233 °C boiling point, depending on who you
ask!
There are other
alkysilanes, and they all seem to have similar boiling points and costs, which
are general affordable for our uses here, so long as we keep our demands for
product purity low, and buy in large volumes.
For glass-associated uses, your other sensible choices seem to be 3-Glycidoxypropyl
silane and phenyltrimethoxysilane.
Note that 3-Glycidoxypropyl trimethoxysilane resin
(commercially known as Dow Corning Z6040) is for glass-to-tape bonding, but
perhaps with a wee tad of reformulation, this could be used for our specific
uses (proposed uses of silane compounds are discussed further below). But Dow Corning sounds like a good place to
start, maybe!
At this point, I would like to expand your
vocabulary with the words grickle, grickled, grickling, and other plausible
variations thereof. Just as sealant, caulk, caulking tool, etc., are generic, in that the precise
formulation of the sealant or caulk isn’t specified, so, too, will be the case
with grickle. Grickle is any formulation
of liquid, paste, or solid for the purpose of being applied to hot glass. It may be applied as anything ranging from a
thin (not very viscous) liquid to a solid, and may be applied as a flux,
bonding agent, coloring or writing application, a molten-glass paint, or a
paint-plus-primer (for hot but solid glass).
Grickle is NOT a word for various formulations of the main glass body
itself! Silane compounds are the first
of several prime liquid ingredients for formulating grickle, especially for
primer or flux applications.
If these (“grickle”) words are adopted in the
glass world, some confusion could be avoided.
For example, “crackle medium” in the glass world is a FAKE glass cover
paint-job, a non-glass paint (resin for example) over real glass. There is no such thing (to my knowledge) as a
single glass layer with REAL glass crackle added, that is fired in as kiln, in
the same sense as there are “crackle glazes” for ceramics (pottery), which are
fired. Crackle glass (all true glass)
today is a 3-layer sandwich of glass; see https://expresstoughening.com/latest-articles/what-are-the-benefits-of-using-crackle-glass/#:~:text=Thanks%20to%20its%20build%2C%20crackle,glass%20in%20a%20busy%20environment. (Cited here several times.) I know of NO “crackle glaze” that is or
contains true glass, applied to one single glass layer and then fired. Invent it, please, and THEN it can honestly
be called “grickle crackle glaze”, to specify that it is intended for hot glass
firing. Perhaps it might work, with some
formulation of (or with) LionGlass applied to glassware (bottles, jars, etc.)
before the glassware is annealed. If so,
the glaze would be called “grickle crackle glaze” as opposed to ceramics-pottery
“crackle glaze”. “Grickle” should
specify hot glass work, and exclude the base layer of glass alone, where the
language is already pretty clear (to me, at least).
“Water
Glass, AKA Sodium Silicate” is also of
interest (and another prime possible constituent of grickle). See https://en.wikipedia.org/wiki/Sodium_silicate . It can serve
as a glue. From
the here-cited link, “Sodium
silicate is also used currently as an exhaust system joint and crack sealer for
repairing mufflers, resonators, tailpipes, and other exhaust components, with
and without fiberglass reinforcing tapes. In this application, the sodium
silicate (60–70%) is typically mixed with kaolin (40-30%), an aluminium silicate mineral, to
make the sodium silicate
"glued" joint opaque. The sodium silicate, however, is the high-temperature
adhesive; the kaolin serves simply as a compatible high-temperature
coloring agent.” (Emphasis mine.) Keep in mind that at high temperatures, as
in, inside (or on the surface of) molten or hot glass, this “water glass” will
outgas. Sodium silicate outgassing temperature
is apparently pretty high. From the
previous link, “Heated to drive off the water, the result is a
hard translucent substance called silica gel, widely used as a desiccant. It can withstand temperatures up to 1100 C”, which is higher than our targeted 871 C for
expanding perlite into LionGlass. Wiki’s
“silica gel” link is https://en.wikipedia.org/wiki/Silica_gel , which says “Silica gel is an amorphous and porous form of silicon dioxide (silica), consisting of
an irregular tridimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores.” So I think that sodium silicate could be
applied (to something, even a glued-together assembly, that you want to add to
molten glass) as a wet glue (and-or paint), then slowly dried and heated, till
it transitions (or partially transitions) to silica gel, and then added to
molten glass. Certainly this could be an
interesting experiment! More on that
later…
What could we do with a glass additive
consisting of silica gel (or pumice), with the internal tiny voids filled with
silane gas? Or silane compounds? Maybe get surface-layer “grit” to bond to hot
glass, to increase friction, if we use glass as a floor tile? I sure don’t know, but thought that I’d
mention it in passing!
“Borax AKA Sodium Borate, or Anhydrous Borax AKA Sodium Tetraborate” is also of interest, and of MUCH interest as a prime ingredient of grickle formulations! https://en.wikipedia.org/wiki/Borax says “Anhydrous borax is sodium tetraborate proper, with formula Na2B4O7. It can be obtained by heating any hydrate to 300 °C.[18] It has one amorphous (glassy) form and three crystalline forms -- α, β, and γ, with melting points of 1015, 993 and 936 K respectively. α-Na2B4O7 is the stable form.[18]" Borax is a water-soluble salt. Note that our above source also says that borax is a “Component of glass, pottery, and ceramics”, and can be used “as a metal soldering flux, as a component of glass, enamel, and pottery glazes.” That sounds REALLY promising for some of the uses proposed further below! It doesn’t specifically say that it could be used for glass-to-glass bonding flux (in hot-glass fusing), but we could be well advised to use borax to formulate (hold together) other compounds that CAN most certainly do this job well!
Note that throughout this entire paper, I have frequently called on borax to be a prime ingredient of “icing” on “sand-apple upside-down cakes”, “glue” for glass-sand sand-paintings on panes of glass, melt-into-the-glass “barriers” between different colors of glass-sands, and so on. Such uses of borax must be “formulated” to “COE-match” the glass in which it is used! So if I don’t repeat that often enough, here it is: Borax contains boron, as in borosilicate glass, which has a low COE, or Coefficient Of Expansion. Many types of “artistic glass” have a higher COE than borosilicate glass has. COE-matching, I am told, is vitally essential in avoiding glass-cracking, if we’re fusing or melting glasses together! So if you (as seems to be often quite likely) need to “jack up” your borax-based formulation’s “COE number”, then add some lead oxide, or other agent! I am frankly no chemistry whiz, and know few more details. Borax is water soluble, and most-effective COE-raising compounds may often NOT be water-soluble… So go with a recently-stirred slurry, maybe, and… Good luck!
Well,
actually, also see sodium carbonate, calcium carbonate, hydrates of the
preceding, calcium bicarbonate, calcium oxide, and associated glass-compatible
chemistry, in hopes of adjusting COE compatibility, as well as “glass fluxing”
action.
“Boric
Acid” MIGHT also be of interest! See https://en.wikipedia.org/wiki/Boric_acid ...
And frankly, the chemistry gets to be “over my head”, and this paper is
too long already! It contains boron,
which is also a pretty useful ingredient of some pretty useful glasses! Enough said, for a glass-amateur like me!
“Calcium Carbonate” is
also of interest. It forms limestone,
stalagmites, and stalactites. Shelled
sea creatures make shells out of it.
I’ve dreamed up some possible uses for it here. See some links and out-takes that I have
collected: https://www.nordkalk.com/solutions/glass-and-sugar/ says… “Limestone (Calcium Carbonate) is one of the main components
in glass manufacturing. Its main function is to introduce Calcium Oxide into
glass recipe, which is needed to improve chemical resistance and durability. It
also acts as flux in glass manufacturing.”
Here’s a slightly different form of it: https://en.wikipedia.org/wiki/Calcium_bicarbonate . Sodium bicarbonate is the form of it that
we’d use if we want it to be water-soluble. See https://en.wikipedia.org/wiki/Calcium_carbonate and https://en.wikipedia.org/wiki/Calcium_oxide too... Never forget the Wiki!
Heat calcium carbonate enough, and you get calcium oxide. For more about calcium oxide, you can see
that it is a common ingredient in glass. See https://www.sciencedirect.com/topics/chemistry/calcium-oxide and https://science.jrank.org/pages/1122/Calcium-Oxide.html and https://engineersblog.net/function-of-lime-cao-in-glass-manufacturing-process/ .
“Sodium
Carbonate” (AKA soda ash) is also of
interest. It is a salt whose hydrates
are water soluble. This is a
soda-lime-glass ingredient. https://en.wikipedia.org/wiki/Sodium_carbonate says that “Sodium carbonate serves as a flux for silica (SiO2, melting point
1,713 °C), lowering the melting point of the mixture to something achievable
without special materials. This "soda glass" is mildly water-soluble,
so some calcium carbonate is added to
the melt mixture to make the glass insoluble.”
“Kiln
Wash” is also of
interest. It is used to prevent hot
(especially glazed) pottery or glass from sticking to kiln shelves or to other
pieces of (art) glass or pottery. Talk
to The Google for more information, of course…
Also see https://kilnfrog.com/blogs/frogblog/ready-set-fire-with-shelf-paper for a ceramic-based (a version of “ceramic paper”)
heat-tolerant insulating “shelf paper” to perform the same function as what
“kiln wash” does.
“Ceramic Paper” is
possibly also of interest, along with other types of ceramics. Note that we are now moving into categories
of often more exotic and expensive materials.
For ceramic paper, see https://dobsongasket.com/products/high-temp-materials for “High Temp Gasket Material”. There, we can see that super-wool (ceramic paper) is rated for up to 1,300
degrees C… Also see ceramics that can bend, at https://www.popularmechanics.com/technology/a19300/new-flexible-ceramic-could-make-its-way-into-electronic-devices/ From
there, “New Flexible Ceramics Could
Make Bendy Gadgets a Reality”
and “The
material apparently has properties similar to paper while retaining the high
heat-resistance of ceramics.”
At further length (from the same source), ‘Eurekite's
"flexiramics" ostensibly retain the positive properties of
ceramics while being flexible rather than brittle. In a video from Eurekite,
CEO Gerard Cadafalch holds a piece of the material over a flame and it doesn't
catch fire. The material can reportedly
withstand heats of at least 1,200 degrees Celsius—about 2,190 degrees Fahrenheit—the hottest temperatures Eurekite's lab can achieve. The company says they can make the material
in thickness ranging from "a few micrometers to over a millimeter,"
according to Phys.org.’
Various forms of ceramics MIGHT
possibly be included inside glass, or incorporated (melded, fused) onto the
surface of glass. More
on that later.
Also
see https://www.researchgate.net/publication/265606808_Strong_lightweight_and_recoverable_three-dimensional_ceramic_nanolattices “Strong, lightweight, and recoverable three-dimensional ceramic nanolattices”.
For a brief discussion of the exact same material, see http://www.sciencedaily.com/releases/2014/09/140911135450.htm “Ceramics
don't have to be brittle: Incredibly light, strong materials
recover original shape
after being
smashed”. Sad to say, I
have found no information about costs or temperature tolerance for this type of
material.
For
a last item on ceramics, the following may be of passing interest to us:
https://www.technologyreview.com/s/411301/ceramics-that-wont-shatter/ . Sad to say, it doesn’t
look to be high-temperature tolerant.
From there, “Because the newly developed
ceramic contains a gluey polymer, it would fail in high-temperature
environments like the inside of an engine. So the Berkeley researchers are
experimenting with metal fillers, which can withstand higher temperatures.” From 2008… A bit old by now!
By now, “ceramic paper” seems to be
readily available. See https://www.ceramaterials.com/ceramic-fiber-paper/ … It’s
a bit pricey, but not exorbitant.
“Google” away for yourself on that aspect of things… To my knowledge, it is available ONLY in the
“color” of white!
“Silica Aerogels” are quite
pricey for general glass-working, so they are only mentioned here in
passing. Carbon-fiber-laden aerogels are
also expensive, but a bit less so than other forms of silica aerogels, and
certainly stronger than many other kinds of silica aerogels. In any case, one could, for example, arrange
an artistic arrangement of glass-compatible sands (especially colored glass
that has been shattered into sand), or other suitable coloring agents, on top
of a piece of aerogel, and then place it onto the top of hot glass. On top of hot liquid glass coming out of a
float-glass machine, for example. The
aerogel would dissolve into the molten glass, leaving behind your artwork, for
posterity! Aerogel with a
white coloring agent (such as suitably-formulated mix with properly-sized
particles of titanium dioxide) would perhaps make some cool-looking
clouds, in glass art!
Iridescence,
Metamaterials, and Stranger Things… Oh my! Excuse me, Dear Reader,
but, late in researching this paper, I stumbled on some more glass ingredients
and techniques, which I will now dump in here, in a probably-disordered
fashion. Ingredients and techniques are
“married at the hips” anyway, right? See
https://en.wikipedia.org/wiki/Tiffany_glass and ALL of what it mentions! “Tiffany glass” is AKA “favrile glass”. An especially noteworthy ingredients-related
set of notes from this “Tiffany” link is now imported: “The term ‘opalescent glass’ is commonly used to describe glass where
more than one color is present, being fused during the manufacture, as
against flashed glass in which two colors may be laminated, or
silver stained glass where a solution of silver nitrate is superficially applied, turning red
glass to orange and blue glass to green.”
See https://blogs.ucl.ac.uk/researchers-in-museums/2019/05/20/the-mystery-of-iridescence-in-glass/ “The
Mystery of Iridescence in Glass”… It seems that at least some of the
iridescence of ancient glass wasn’t there originally, but was picked up over
time and aging. Along the same lines,
see https://thedebrief.org/2000-year-old-substance-unearthed-at-ancient-roman-sites-possesses-extraordinary-properties/ and
similar recent articles regarding “aging in the mud” that created “photonic
crystals” over time. “Wow glass” makes a
good search-string for more information.
Perhaps if materials scientists can figure out how to speed up (and make
affordable) these kinds of glass-aging processes, we can make beautiful
art-glass butterflies, for example! If
not integrated into the glass, then perhaps in art-glass “cookies” (see much
further below, concerning that).
Parenthetically,
let me interject, look carefully at any ads or web sites about “iridescent
glass coatings”, which will (Usually? Always?) be non-glass paint jobs…
NOT embedded deep in (and through) the glass, like Tiffany glass. Non-glass “paint jobs” will be less durable
than “dyed in the glass” jobs, of course.
They might even peel right off, over time!
“Glass metamaterials”
makes a good search string, as does “Bragg Stack”, or “dichroic glass coating”.
Concerning the latter, see https://en.wikipedia.org/wiki/Dichroic_glass and https://cbs-dichroic.com/ …
Who knows what future “tech” will bring to the artistic
glass-working world?!
“DNA-Based Glass and Other Exotic Materials” are
possibly also of interest, for those interested in such things… I imagine that some (if not all) of the
following materials will be WAY too exotic and costly for most of the uses that
I will describe. AND my imagination is
failing to come up with any suitable applications for such exotic and
doubtlessly high-cost materials! But
here are some such choices…
https://scitechdaily.com/scientists-create-new-material-five-times-lighter-and-four-times-stronger-than-steel/ … This concerns DNA and nano glass covering the DNA with empty
spaces left… “Scientists Create New
Material Five Times Lighter and Four Times Stronger Than Steel”. From there, “The team plans to look at other materials, like carbide
ceramics, that are even stronger than glass to see how they work and behave.”
The
following may be less exotic and expensive than the above. Let’s call these “glassified fibers” for further-below reference. See https://scitechdaily.com/a-new-way-to-control-fire-scientists-develop-novel-nanoscale-material/?expand_article=1 “A New Way To Control Fire – Scientists Develop Novel Nanoscale
Material”. Here, a thin glass layer
develops over the outer surfaces of cellulose fibers. And then this glass
prevents quite as much oxygen from getting in to oxidize the fibers…. Out-take below:
“Here’s how ITD works. You start out
with your target material, such as a cellulose fiber. That fiber is then coated
with a nanometer-thick layer of molecules. The coated fibers are then exposed
to an intense flame. The outer surface of the molecules combusts easily,
raising the temperature in the immediate vicinity. But the inner surface of the
molecular coating chemically changes, creating an even thinner layer of glass
around the cellulose fibers. This glass limits the amount of oxygen that can
access the fibers, preventing the cellulose from bursting into flames. Instead,
the fibers smolder – burning slowly, from the inside out.
“Without the ITD’s protective layer,
applying flame to cellulose fibers would just result in ash,” Thuo says. “With
the ITD’s protective layer, you end up with carbon tubes.”
The above links to here: https://onlinelibrary.wiley.com/doi/10.1002/anie.202308822 ... From there, “Herein, alkysilanes grafted onto cellulose fibers are
pyrolyzed into non-flammable SiO2…” So silane, as
previously mentioned here (alkysilanes more specifically),
are a “magic ingredient” for us! Or at
the very least, a prime candidate ingredient!
More specifically, my further research tells me that the specific alkysilane
here (or certainly, one of great interest to us) is likely to be phenyltrimethoxysilane. More about that later.
As best as I can tell, the following
is still not available for sale. It may
be too exotic. See https://www.mpg.de/1166083/heat-resistant-ceramic for heat resistant ceramics that can be
drawn into fibers. These might fit in
here as well. From there, “Ceramic
fibers made of silicon, boron, nitrogen and carbon
remain tough and stable even at temperatures above 1500 degrees Celsius.” https://www.sglcarbon.com/en/ (SGL Carbon) in Wiesbaden, Germany, apparently
MIGHT someday still plan to start producing these fibers, and they may prove to
be suitable for building high-temperature-tolerant composites, which are NOT as
brittle as ceramics or glass usually are.
Or perhaps such fibers could be embedded inside glass, or we could use
them as paint bristles for painting liquid glass onto a base layer of
glass. Time may tell about price and
availability.
This concludes my list of exotic or
semi-exotic materials candidates for possible use with LionGlass. Some other simple non-exotic materials will
be mentioned further below, but I don’t want to add too much clutter here to my
materials “palette” section.
Well, let me amend that thought with
another thought! In a sort of an
in-between fashion, let me mention…
“Embedded
Wires or Mesh” inside the glass has
historically been used to create an older kind of “safety glass”. https://www.gindeglass.com/wholesale/wire-mesh-glass/ says
“Wire mesh glass (also known as Georgian
Wired Glass) has a grid or mesh of thin metal wire embedded within the glass.” http://www.electrontubestore.com/index.php?main_page=product_info&products_id=1191
is a good source for wires made of
Kovar, to make a mess well-matched (CTE-wise) to borosilicate glass. As previously mentioned, I have no idea what
the coefficient of thermal expansion (CTE) of LionGlass is, so I don’t know if
Kovar is a good fit, or not, here, but maybe we could make our wire mesh out of
Kovar wire. https://www.glassmagazine.com/article/wired-glass says “Monolithic wired glass is rolled, polished, annealed glass with a layer
of wire embedded in the middle. Historically, wired glass was used in locations where an ‘appearance’ of
security was sought, for example, in jewelry display cases and museum displays.
It has also been used in skylights to prevent broken glass from falling into a
room or onto walkways in the event of breakage. Wired glass gained popularity,
in part, because it can be cut to size from stock sheets in the field, using
tools commonly used for glass cutting. The primary use of wired glass was and
continues to be in locations that require fire-rated construction materials.”
What kind
of wire (using what kinds of metals) is used inside “”wire mesh glass”, AKA (Also
Known As) “Georgian Wired Glass”? My research tell me that stainless steel, copper, brass, aluminum alloy, or steel can be
used. Kovar might possibly be more
expensive than what is really-truly needed here, much of the time.
Why do I
bring this (wire mesh) up here? Not so
much for “safety glass”, but more-so for “artistic glass”. Wire mesh might be used to suspend other
items to be embedded into “float glass”, for example, to create artistic forms
of flat (plate) glass. More about all of
that later, alligator!
“Miracles Happen Here Formulations” is a
short section that will help us to transition out of a materials palette,
towards techniques, tools, and wild speculations of mine. Not being a chemistry whiz OR a glass expert,
I’m sure that I’ll make some mistakes!
But here’s a list of formulations that I’d like to see being made to
work, and wild guesses as to their likely ingredients, with more details
further below. Sometimes I call them ingredients of glass-working “grickle”, a
word that I’ve made up, in hopes of being helpful!
Flux for adding splotches of color to glass-ware… A base layer or primer layer added to glass
(think bottles etc.) before annealing them.
Over this base layer goes a (hopefully COE-compatible but maybe not well
matched; colored-surface-layer “crackling” might be OK) layer of colored liquid
glass. The liquid glass might have a
lower melting point than the base glass.
Anyway, flux or primer might contain solids (metal oxides for fluxes
and-or coloring agents) suspended in a liquid or slurry. Stir often if needed. The liquid base might contain silanes, hydrated borax, boric acid, sodium carbonate
hydrates, water glass, calcium bicarbonate, any of various forms of “grit”, or some
kind(s) of short fibers. Insert magic
here! Oh, and, the primer might somehow
be combined with a glassy coloring layer, perhaps… OR on the other hand, MULTIPLE layers of
primers and glass paints might be needed!
(Temporary) glue for holding colored sands (in
an artistic arrangement) on top of a plate of glass, such that the sands aren’t
disturbed or “jostled” while being moved and processed, before being
“fused”. The exact same list of suspects
(materials candidates) as listed immediately above can re-apply! Since the purpose is different, I suspect
that the formulation will need to be different.
Once more, the glue just might be applied in multiple layers, including
before and after the colored-glass sands go down. As usual, we’ll want to pay attention to COE
(Coefficient Of Expansion), and hopefully not polluting, too much, our
nitrogen-hydrogen environment (if applicable), as our glue out-gasses (while it
gets burned off and-or incorporated into the glass product).
“Icing” on the “sand-apple upside-down
cake”, AKA, the thumped-brush method. See details further below. Once again, the
same candidates and criteria apply. The
job is much tougher this time, and it may not be possible! The dried-out formulation must cling to the
brush-bristles just right… Not too
strongly, and not too weakly!
“Dissolvable stitches” for
glass. Search for “stitches”
far below, for context. Most of the
above comments apply once more, but with more emphasis on fibers, I would
think. Maybe thin metal wires as well.
Glass-Working Techniques
“Slumping Glass” is one
method or technique. See glass slumping
technique and forms at. https://www.thecrucible.org/guides/glass-slumping/ . Here, we learn that most
molds (for slumping glass) are made of ceramic clay or stainless steel. Now the following link is a repeat from
further above, but https://www.glass-international.com/news/lionglass-a-low-carbon-step-towards-a-sustainable-future#:~:text=Soda%20lime%20silicates%20have%20a,of%201400%2D1500%C2%B0C.&text=Considering%20that%20carbon%2Dbased%20energy,carbon%20footprint%20of%20its%20own tells us that “…soda lime silicate reaches its softening point, the point
where it begins to slump underneath its own weight, at about 730°C.8 LionGlass
reaches this same point more than 100 degrees lower, at about 590°C.”
Google at will as usual, of course, but my research has told
me that one can soften glass at a lower “slumping point” v/s a higher “fusing
point” where glass melts together. If you’re
a glass artist who wants to fuse one piece of glass together with another one,
and THEN slump the two, do your fusing first, cool the assembly down, and then
do your slumping, second. Soda-lime
glass fusing point is here: “Soda-lime glass fuses between 1350
degrees Fahrenheit to 1500 degrees Fahrenheit.” . This is according to https://www.thecrucible.org/guides/glass-fusing-3/#:~:text=What%20temperature%20do%20you%20fuse,higher%20than%20soda%2Dlime%20glass. That translates to
732 to 815 degrees C. LionGlass will
probably fuse (together with LionGlass) at 100 C or so lower than that. Fusing one type of glass to another type of
glass is an unknown for me, especially concerning LionGlass! This is mostly since I don’t know what the
“COE” of LionGlass is. But I can (and
will) always speculate!
“Float Glass” is also of interest. See https://en.wikipedia.org/wiki/Float_glass . Rather than cluttering up this paper with too
many easily-available out-takes, let me just summarize that the glass is
“floated” on molten tin, in a nitrogen and hydrogen atmosphere. Nitrogen (an inert but affordable gas) I can
understand, to keep the tin from oxidizing.
I would speculate that the hydrogen is added so as to absorb (combine
with) any oxygen that manages to sneak in.
In chemistry-speak, this is: Hydrogen is added to insure that we have at
least a slightly “reducing” environment, not an “oxidizing” environment, with
some “buffering” or safety margin added, beyond merely providing an inert-gas
environment. LionGlass MIGHT be
“floated” on a lower-melting-point liquid metal as compared to tin, but I would
humbly submit that the price of such a development process (for some other
molten metal) wouldn’t be worth it. I
have been taught that “If it ain’t broke, don’t fix it!” Also note that the glass is slowly cooled in
a “lehr”, to “anneal” the glass. See https://en.wikipedia.org/wiki/Lehr_(glassmaking) and then also https://en.wikipedia.org/wiki/Annealing_(glass) . I don’t know if LionGlass will be any more
tolerant of rapid cooling (in need of slow annealing), compared to other types
of glass, but I would dearly love to know!
This is (especially economically, and in terms of environmental impact)
very IMPORTANT!
OK then, not TOO much clutter here,
hopefully, but also see https://www.sciencedirect.com/topics/engineering/float-glass-process#:~:text=In%20the%20float%20glass%20process%2C%20the%20ingredients%20(silica%2C%20lime,of%20a%20molten%20tin%20bath. From
there, “In the float glass
process, the ingredients (silica, lime, soda, etc.) are first blended with cullet (recycled broken glass)
and then heated in a furnace to around 1600°C to form molten glass. The molten
glass is then fed onto the top of a molten tin bath.” (Emphasis mine. Cullet… Expand your
vocabulary!) Also, from https://www.guardianglass.com/eu/en/our-glass/glass-types/float-glass ,”In North America for example, the ribbon thickness can range
from approximately 3/32” to 19/32”. The typical width of the float glass ribbon is 102 inches,
and the maximum length is 240 inches. In Europe, the ribbon thickness can range
from approximately 2 mm up to 19 mm.”
The “ribbon” refers to the molten glass that is floated out, to anneal
and cool, forming large panes (sheets) of solid glass, which are then cut and
processed.
Now, concerning the following topic (and link), I will
DEFINITELY want to speak to this some more, but note that for glass at the very
least, the “annealing” and cooling processes will take longer, the thicker that
the material (glass) is. Else we have
differential cooling on the surface v/s the inner parts of the material, which
leads to differing thermal contraction due to cooling, and hence, to cracking
(or sometimes surface “crazing”). See https://www.space.com/giant-magellan-telescope-mirror-final-mirror-casting and
from there, “This last
mirror will take four months to cool.”
This is FOUR MONTHS of annealing and cooling time! Well OK, perhaps more precisely, https://giantmagellan.org/2021/03/05/engineering-marvel-sixth-mirror-cast-for-giant-magellan-telescope/ says “The
mirror then enters a one month annealing process where the glass is cooled
while the furnace spins at a slower rate in order to remove internal stresses
and toughen the glass. It takes another 1.5 months to cool to room
temperature.”
“Foam
Glass” is also of interest. See https://en.wikipedia.org/wiki/Foam_glass , which tells
us that “Foam glass is a porous glass foam material.” Also
from there, foam glass has “…a small expansion coefficient (8
× 10 °C)…” It has dimensional
stability, that is. I see no reason to
recite or repeat more facts about it for now.
More about “foam glass” later, though (well, maybe in
another paper, for MUCH more about it; not much more for now).
Materials Behaviors of Glass (and Ceramics)
“Annealing
Glass” is one thing we’ve already
discussed, or at least, mentioned.
Glass, compared to many other materials types, requires a LONG time to
cool down from the molten state, if you don’t want it to crack! And the thicker it is,
the longer that the required cool-down time is.
Technically, not ALL of the cool-down time is “annealing time”. And specifically with
respect to LionGlass on these matters?
I have NO idea, but would LOVE to know!
“Crazed Glass or Crazing, and Cracking” are other
concerns. Take your work of art (or
utility, or both) out of the kiln (and cool it down) too fast, and it will
develop deep cracks and-or surface cracks, or become “crazed”. There can be other causes as well. See https://stoveglassdirect.com/crazed-stove-glass/#:~:text=Crazing%20refers%20to%20small%20hairline,way%20you%20operate%20your%20stove for another example of a cause of “crazing”
in glass. I’m not totally sure about the
following matters, and don’t want to get side-tracked too much here, but I
think it is safe to say that surface “crazing” is more acceptable in the
pottery (ceramics) world, than in the glass world, both artistically and
structurally. In pottery, “crackle
glaze” can be used to attain this effect deliberately. Designed “crackle glass” is a “thing” as
well, it seems. See https://expresstoughening.com/latest-articles/what-are-the-benefits-of-using-crackle-glass/#:~:text=Thanks%20to%20its%20build%2C%20crackle,glass%20in%20a%20busy%20environment.
… And we learn that 3-layered
glass… Two surface layers of unbroken
glass, and a middle layer of cracked glass… Can actually be QUITE safe
and strong. “…crackle glass is up to six times stronger than regular glass panes…” says
this web site. Single-layer “crackle
glass” is always (to my knowledge) PAINT on glass, not solid glass, or a
high-temperature-fired glaze, as can be used on pottery. See https://digitalfire.com/glossary/crackle+glaze#:~:text=Crazing%20is%20a%20crack%20pattern,ware%20fired%20at%20low%20temperatures , which says that “Crazing is a crack pattern caused by thermal expansion mismatch between body and
glaze. After the glaze solidifies (as the kiln cools) it shrinks more than the
body. To relieve the tension of being stretched, it cracks. Crackle glazes are
typically found on ware fired at low temperatures. Stains and other colorants are often rubbed into the crack lines to heighten the effect.” See https://digitalfire.com/glossary/crazing as well. AND see https://marjonceramics.com/pages/Tips/tip14.htm#:~:text=Thermal%20shock%20can%20result%20when,result%20in%20cracking%20or%20breaking , “CRACKING AND THERMAL SHOCK”.
It is highly probable that the word “craze” should NOT be used when
“cracking” is meant. Actually, I find
some conflicting messages “out there”!
See https://bigceramicstore.com/pages/info-ceramics-tips-tip40_cracking_crazing_shivering_dunting also. Now let’s move on!
“Crizzling Effect in Glass” is a
concept that could potentially be confusing with cracking or crazing, but is
different. Apparently it refers to
surface cracking (and even loss of surface materials) in poorly formulated or
processed, often older or antique, glass, due to
intrusion or incorporation of moisture (water).
See https://www.cmog.org/article/use-equilibrated-silica-gel-protection-glass-incipient-crizzling , “THE USE OF EQUILIBRATED SILICA GEL FOR
THE PROTECTION OF GLASS WITH INCIPIENT CRIZZLING”
“Meniscus Effect in Glass” is a perhaps-wild
question or speculation on my part. In
water, the inter-molecular bonds that are normally fully 3-dimensional are
forced to go semi-2-dimensional at the surface of the water (bonds are
concentrated), forming a higher-strength surface. Some people say that even room-temperature
glass is a VERY-VERY THICK (viscous) liquid.
See https://www.desy.de/user/projects/Physics/General/Glass/glass.html#:~:text=Conclusion,is%20neither%20liquid%20nor%20solid
. Does cold “thick-liquid glass” have a
stronger surface layer, a similar “liquid” meniscus? I have “Googled” a LOT, and have no firm answer,
with respect to LionGlass! Does ANY form
of today’s commonly used glass show this “meniscus” behavior? (There, I believe that the answer is a fairly
firm “no”.) Will LionGlass show it? I would dearly love to know!
If so,
if LionGlass will show the meniscus effect, this effect should strengthen in a “foam
glass” version of LionGlass, to the point that foam glass has more strength
per-mass-of-glass than the solid glass does.
Emphasis here is on PER MASS of glass!
Would added strength also show up at the interface between LionGlass and
presumably lower-density inclusions, such as expanded perlite? I should hope that these questions are at
least worth asking…
For
LionGlass specifically, if there is no answer yet, to this question (is there a
meniscus effect at work here), a suggested testing technique is as
follows: Use some solid masses of
LionGlass (say 0.1 Kg), and test their strengths to destruction, both in
compression and tension loading modes. Now
create some foamed-LionGlass specimens of 0.1 Kg as well, and test them
likewise. If the foam glass is
significantly stronger PER MASS, then the meniscus force is likely to be at
work!
Possible New Tools for Glass-Working
I now
want to move towards describing a proposed tool that I’ll call a “hot-glass
gun” (evoking a
hot-glue-gun). But first, I want to
describe associated ideas, sub-components, and accessories. Unlike a hot-glue gun, handling a working hot-glass
gun in human hands, even in thickly gloved human hands, sounds prohibitive for
anything other than the briefest snatches of time! For optimal, sustained use, such a tool will
be used mostly inside a hot kiln, remotely controlled by humans or machines
(especially by robots), I would think.
“Silicon Carbide
Electronics” is a relevant topic, if, as seems highly likely, we’ll need some electronics located inside a hot
kiln. These are well known to be
high-temperature tolerant. See https://www1.grc.nasa.gov/research-and-engineering/silicon-carbide-electronics-and-sensors/ or
any of MANY other “Google hits” about this…
Silicon carbide for electronics is a well-known idea. What is a bit more of a narrow subset of this
may be electronic high-temperature memory, where we have this: Deep Jariwala (a researcher) and scandium
aluminum nitride … See
https://www.newscientist.com/article/2394256-super-heatproof-computer-memory-survives-temperatures-over-500c/
(sorry, it is pay walled)… But from
there, “Super-heatproof computer memory survives temperatures over 500°C; A kind of computer
memory made from the semiconductor scandium aluminium nitride withstands
extreme heat in tests, making it potentially useful for space missions”. This is a just-FYI lead for deeper research
for those interested in such things…
Please be advised that the “New Scientist” full article isn’t very long
or detailed, at all.
“Ice-Boxes Inside the
Kiln” are a good idea to chill (at least a LITTLE bit) high-temperature
electronics, motors, sensors, cameras, and other gear that really should
optimally be located inside the kiln. Yes,
locate as much gear as you reasonably can, OUTSIDE of the kiln, and relay data
via cables and fiber-optics, and forces via shafts, gears, chains, and cables,
if you can. The rest
of them? Protect them from heat,
while being located inside the kiln! A
bit of “leakage” of cooling fluids routed to the “ice boxes”, into the hot kiln
environment, can be tolerated. And yes,
“ice boxes” could be located inside hot-glass guns, which are, in turn, located
inside the kiln. See “ice-boxes” (use
that as a search-string, or just “ice box”, and see Figure #1) at https://www.researchgate.net/publication/355039141_Harvesting_and_Managing_Energy_While_Re-entering_an_Atmosphere_Using_a_Shuttlecock_Design . This paper in turn cites another paper, which
discusses a useful constituent ingredient for building such a heat-shielding
“ice box”, which is at https://www.researchgate.net/publication/331556573_Designs_for_Passively_Thermally_Gated_Fluid_Flow_Switches (and also
at http://www.rocketslinger.com/Psv_Tgt_Fsw/ ), titled “Designs
for Passively, Thermally Gated Fluid Flow Switches”.
“Hot Cameras” would be useful inside a
kiln, or inside a float-glass factory, both for remote control by humans, and
for AI-driven robots observing (“learning”) the processes. And perhaps eventually for controlling the
processes! Since a float-glass factory
contains an atmosphere of nitrogen and hydrogen, placing a human in there
becomes prohibitive! So “spy cameras” to
watch these processes sounds like a good idea!
(They may already exist; I don’t know.
But here are some ideas.) Stationary
cameras are less troublesome; Mobile cameras would be more complex. In either case, high temperature exposure
will probably be troublesome. If not in
short time-frames, then more likely, long-term heat exposure will degrade
materials eventually. So we’ll want to
insulate and cool the most sensitive, vulnerable parts… Camera, wires, cables
(perhaps), and fiber optic cables.
See “ice boxes” in this document.
A high-pressure cooling fluid could be routed (along with wires, cables,
etc., to keep them cool as well) through successive-pressure-dropped stages,
making sure that the last (camera) stage stays cool. The final camera and camera-eye could be
protected by “quartz glass”, AKA near-pure silica glass, which is expensive,
but quite heat-tolerant. Use true quartz
glass (amorphous), and NOT crystalline quartz.
This is per the following: https://www.crystran.co.uk/optical-materials/crystal-quartz-sio2 says (with respect to crystalline
quartz, not quartz glass) that “Quartz should
not be processed or used at temperatures greater than 490 °C” So, for this particular application,
use quartz glass; quartz crystals are ruled out!
“Study
up” on fiber optic borescopes as well; there are applicable ideas involved
therein. If you want to actively
maneuver your camera around, I am out of novel (but plausible) ideas to
suggest. Manipulator cables (strings) as
are used to make stage puppets dance, might be a good place to start.
“A Hot-Glass Gun”, I
consider to be a prime feature of this paper.
Some people have even been known to say that “happiness is hot,
hot-glass gun”, while others say that saying such things is a bit low and Beatle-browed. I’m staying OUT of that fight!
A
hot-glass gun would have an outermost shell to contain all of the rest of the
gun. Then there would be a cavity
containing a heat source, which could either be electrically-heated wires or
coils (nickel-chromium is commonly used), or gas-fired flames. Both kinds of heat sources are used in kilns,
but electric power is usually best, for easy temperature control, and for other
reasons. The drawing(s) here will show
flames, to make things intuitive.
Inside
the heater cavity, there will be another innermost cavity containing the
feedstock. Feedstock could be called
“glass mash”, perhaps. It could be raw
ingredients (sand, etc.), or it could be previously-formulated glass, which was
smashed to bits to create glass-sand.
“Glass mash” ingredients could be fed into the tool hot or cold, liquid
or solid. And it could be routed into
there (through both the outermost tool-wall, and the inner wall of the heating
cavity) in 1, 2, or more channels (or pipes, ducts, etc.), for different
ingredients, to include glass, coloring agents, additives like perlite, etc.,
at different spots along the tool, as the “glass mash” travels towards the
hot-glass dispensing tip of the tool.
Ingredients could be conveyed (pushed) by Archimedes Screw augurs, into
the glass mash. The glass mash (in its
innermost cavity, inside the heating cavity), in turn, is propelled towards the
dispensing tip by TWO funnel-shaped rotors, which rotate against each other (while
agreeing with each other at the contacting, mating interface between each
other, direction-of-spin-wise), in the style of eggbeater rotors. The beating blades of a conventional
eggbeater are far, far too long (extensive, hard to drive through a thick,
viscous glass mash), though, and should be replaced by simple, short rods. The dual rotating rotors would probably best
be equipped with Archimedes Screws as well (along with the short rods), to help
propel the mash, along with the mixing-grinding forces provided by the rods. The rods are there to help force mixing. With short rods, it would ALSO actually be possible
to make the mating surfaces of the rotor DISAGREE with each other (where
spinning surfaces meet), if the rods are suitably located, and this design
would then force even more vigorous mixing.
It is my opinion that such a design choice is too aggressive and
power-hungry, for the thick “glass mash” involved here.
It is
high time, now, to start providing drawings.
Here’s a drawing of a single rotor for a hot-glass gun. I think that a funnel shape is optimal,
although the degree of taper could be changed.
It could even be taken down to ZERO taper, where we’d have two
pie-dough-rollers rolling against each other.
The size of the device is unspecified.
Suit yourself!
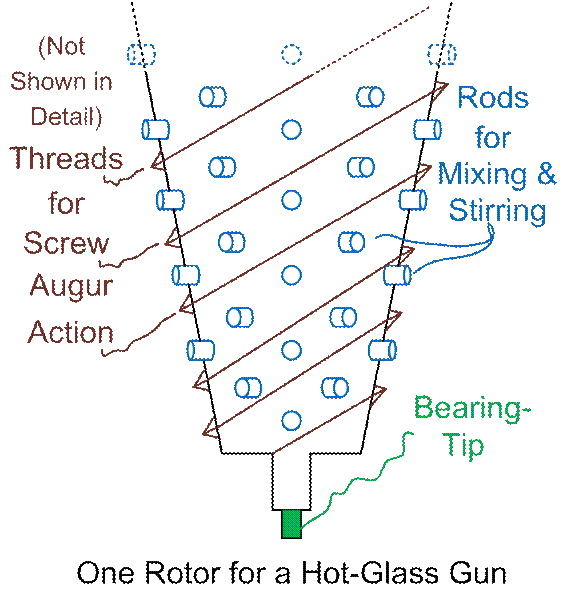
Figure #1
Next, let’s zoom out, and lose tight focus on
the rotors…
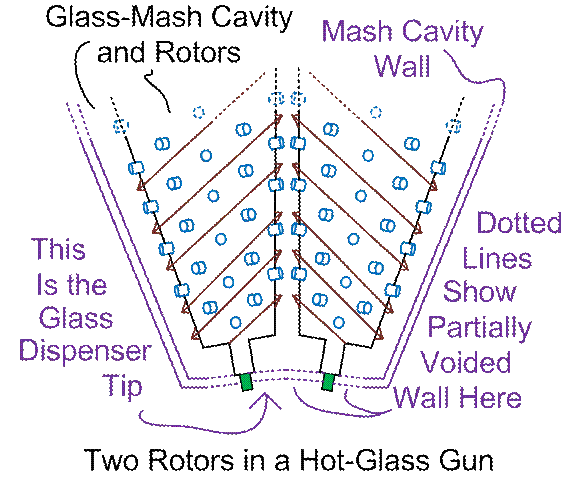
Figure #2
Next, let’s zoom out some more, and lose more
label-clutter on the innermost parts…
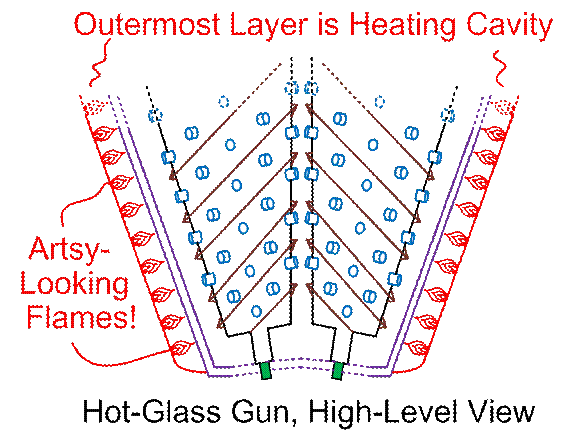
Figure #3
Some more notes are in order. Note that I used double purple lines for the
walls of the glass-mash cavity, just to make it intuitively clear that some
thickness is in order here, not only to contain the rough, muscular nature
(depending on precise uses here) of munching and crunching on glass fragments
and-or thick liquid glass, but also, to properly constrain the
bottoms-of-the-rotors bearing-tips. The
outermost tool layer (the outer single-lined red wall as shown, outer wall of
the heating layer) are shown in a single red line, simply because I was
lazy! If the hot-glass gun is going to
be operating at temperatures significantly higher than its environment (the
kiln or float-glass factory), then yet ANOTHER layer (not shown) should perhaps
be added, and that would be some thermal insulation.
Also
not shown are feed lines feeding the ingredients to the glass mash. As previously
remarked, both the heat-wall and the mash-and-rotors-cavity-wall will need to
be penetrated by the augur-driven feed lines, with motors or drive shafts for
the feed line augurs. Drive motors and-or
drive shafts for the mixing rotors are also not shown. Or “ice boxes” to protect some of these
things! Use your imagination and
mechanical skills, and GO for it!
Now
think also, about humans (and most other mammals) having jaws that allow a bit
of “float”, or flexibility, to mash and chew on harder foods. Too much rigidity isn’t good! For that reason, the “top” ends of the two
rotors (not shown here, but on the opposite ends of the rotors, away from the
dispensing nozzle ends) might want to have spring-loaded shaft-bearings, to
provide some “slop” (a more technical term being “compliance” I believe) to
allow the tool to flexibly munch-and-crunch on chunks of glass, or other solids,
or stiffer “lumps” in the mix, which might be too “thick”. More-compliant bearings there might make more
sense than having more-compliant bearings on the (as shown here) dispenser
tip. Mixing and grinding forces
generated by “chunks” in the mash, should be much diminished by the time the
glass-mash reaches the dispensing tool-tip.
If the tool gets stuck and won’t “go” (rotors won’t spin), because of a
large chunk or lump of glass, or for some other reason, spin-sensors and some
software “smarts” should be added… It
won’t go? Reverse a bit, and go at it
again! Apply retries quantity “X”,
before, perhaps, a longer pause time, or a total stoppage of the gloppage! (“Gloppage” being a technical term for gloppy, gloopy, thick,
viscos, perhaps even lumpy molten glass, in case you weren’t aware of that.)
Another
important point DEFINITELY deserving mention here is that the entire glass-mash
cavity could be PRESSURIZED (with respect to the kiln or float-glass environment,
of course) to help propel the glass-mash towards the dispenser tip. Plain old air may work, if oxidization of
mash ingredients, or other items or materials in the kiln, isn’t a
concern. Or use an affordable inert gas like
nitrogen. Or perhaps nitrogen mixed with
hydrogen, as was previously mention here with respect to https://en.wikipedia.org/wiki/Float_glass .
A
“business end” view of the hot-glass gun may be in order. This focuses on the voids in the glass-mash
wall (and the bearings there) for the hot-glass dispenser tip.
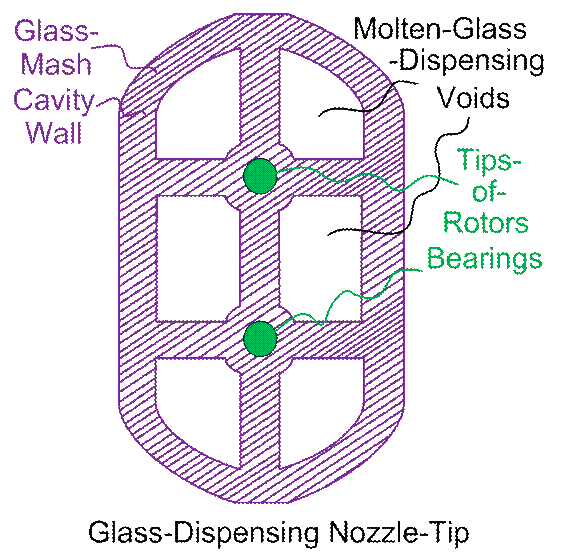
Figure #4
Materials for this “hot-glass gun” could be
anything heat-tolerant, strong, and affordable enough. I would say that stainless steel, titanium, and-or silicon carbide (or a mix
thereof, with appropriate materials at appropriate locations) would be wise
choices. Exotic (and expensive, no
doubt) materials that could be considered would include materials from https://www.sglcarbon.com/en/ ; See https://www.sglcarbon.com/en/markets-solutions/material/sigrabond-carbon-fiber-reinforced-carbon/ more specifically for carbon-fiber-reinforced carbon, for
example.
https://www.blastone.com/product-category/blasting/blast-nozzles/ says that silicon carbide is a good choice for sandblaster
nozzles. Our hot-glass gun-nozzle
(depending on what materials are dispensed) may be subjected to many erosive
forces as well (just like a sandblaster), so silicon carbide might be a good
choice at our nozzle. Note that silicon
carbide has a decomposing point at 5,130 F or 2,830 C, which leaves us with a
LOT of margin (at least for the uses that I envision).
Note that many sorts of variations could be designed, differing from what has been diagrammed and
discussed so far. For example, the
rotating shafts of the mixing-grinding rotors could be capable of flexing. The glass-mash chamber (containing the two
rotors) could then be elongated at the “tops” of the drawings (with little or
no taper “up” there), the rotating drive shafts could change their angles, and
only the last jaunt (of material flow) to the dispensing tip would then be inside
a tapered tool. A flex-shaft joint is
shown here: https://maedlernorthamerica.com/wp-content/uploads/2020/09/PWN_N-1024x601.jpg . Here’s another
photo https://4.bp.blogspot.com/hTt5KshO4DsM/WXxvRS0YWRI/AAAAAAAAABA/Nc_RUXSIEf8rktfjsz6OTNHjsQPRUM4lwCLcBGAs/s1600/34-smooth-x-34-smooth-forged-u-joint-fr2630.jpg . An upstream
umbrella-like “shield” could be installed inside the mash chamber, to at least
partially protect the flex-joints from way-excessive erosion. Wherever such flex-joints exist, mixing and
grinding would be minimized in a bit of a “holding chamber”.
Also note that feed-stock-feeding lines (not shown in
drawings here at all) could be located at any convenient spots along the flanks
(length) of the tool, or at the materials-flow-starting BASE (off-screen “top”
as shown in drawings here) of the tool. And, concerning the (in the above drawings)
blue-colored short mixing rods protruding from the rotors? Such rods could, of course, be many or
few… And they could ALSO be pointed
inwards from the mash-cavity walls, as well as outwards from the rotors. As for me, my personal opinion is, don’t “do”
any over-kill! More of these means more
force is required to drive the rotors, and our
mash-mix will be quite THICK (viscous).
Many other variables are obvious, and so, will not be
discussed here.
Simpler Versions of This Tool could be ultra-simplified…
One could simply ladle highly molten liquid glass into a hollow tube
with a constricted end, writing-pen-style, and have the liquid be
gravity-fed. I think that this idea is
HIGHLY implausible, simply because of how “thick” molten glass is, even at very
high temperatures. This idea becomes
only SLIGHTLY more plausible if, after loading the liquid contents to the
“glass pen”, we add a connection to pressurized gasses at the feed end of the
pen, to blow the molten glass out, creating a “glass-dispensing pen”, or
“grickle pen”. Or a solid pusher-rod,
syringe-style. A pipette-style
dispenser, with a flexible bulb that can suck up the molten glass, and then
dispense it, is, I think, clearly implausible.
For starters, such flexible but heat-tolerant materials as would be
needed for the “squeeze bulb” of a pipette simply don’t exist. A spring-loaded “draw-off syringe” would be a
LOT better alternate for such a thing, in this application.
An outermost “heating layer” could more plausibly be done
away with. Going from two mixing rotors
to just one is also highly plausible, especially if we add at least a few mixing
rods (shown above in blue) to the outer wall of the mash chamber, as was
discussed a bit above, in this paper.
Paint Brushes for Liquid Glass may sound absurd at first glance, but painting a liquid glass
with a lower melting point (such as LionGlass) onto some other type of
still-hot-but-mostly-solid glass, and hoping for the two types of glass to
durably “fuse” to a useful degree, may make sense. This will work if the chemistry works! Sad to say, I don’t know for sure if it will, or
not. Are there hot chemicals which could
be brushed on as a “primer layer” to assist the fusing action of two types of
glass? Apply a primer, before adding
(painting or otherwise) a layer of LionGlass?
Probably! Let’s save that for
later. It gets rather complicated. But please note that as we move away from the
“heavy guns” (hot-glass guns), which I envision as being used to lay down
entire layers of glass (base glass), towards the simple tools, which I envision
being used on selective areas as a coating, we can speak, now, of “grickle
tools”. Brushes for hot glass are
clearly “grickle tools”.
For now, let’s say that the bristles for such a
high-temperature grickle brush could be metal, strips of “ceramic paper” (use
“ceramic paper” here in this paper as a search-string), ceramic fibers, or
high-tech, doubtlessly-expensive fibers (as were already previously discussed)
from Germany, at (a repeated link here) https://www.mpg.de/1166083/heat-resistant-ceramic for heat resistant ceramics that can be
drawn into fibers. These might fit in
here as well. From there, “Ceramic
fibers made of silicon, boron, nitrogen and carbon
remain tough and stable even at temperatures above 1500 degrees Celsius.” (End of repeated section). Note that “ceramic paper” as is formulated
today, most likely wouldn’t be suitable here, since it won’t absorb liquid
glass.
Metal bristles may be subjected to too much
bending and “metal fatigue”. They might
disintegrate too soon to be practical, that is.
On the other hand, if the tips of metal bristles stir into the
underlying thicker (harder) glass layer, while the thinner cover (“painted”)
layer of glass is applied, this may help to strengthen the bond, by intermixing
glass types. If used in an oxygenated
environment (a kiln containing air instead of inert gasses), the oxides layers
of metal bristles, as the oxides are formed and then abraded into the glass,
may also serve to strengthen glass-on-glass bonds (via molecular-bonds-networks
formation), via “fluxing” action, and by lowering the melting point of the
surface of the underlying solid or semi-solid glass. Depending on WHICH metals are used in the
brush-bristles, that is, for the chemistry aspects of bristle-surface oxides!
One could also intermix or blend the bristles-types. For example, strips of ceramic paper could be
curled into “C” shapes, to increase bristle stiffness and molten-glass-carrying
surface area, with the base (but only the base) of the “C” shapes enclosing
wire bristles. The “C” shapes could be
completed to form tubes, almost-tubes, or slightly-doubled-wrapped tubes of
this “ceramic paper”. One might even
force-feed the head of a hollow-handled brushing-tool with the thin (hot)
LionGlass, or LionGlass-plus-additives, molten-glass “paint” to be painted onto
the harder (and perhaps cooler) surface to be “painted”. And obviously, if we chose to use strips of
ceramic paper, we want to choose or formulate such “paper” to absorb, rather
than repel, molten glass... If this is
possible! Shelf-lining ceramic paper
(previously mentioned as an alternative to “kiln wash”) would presumably NOT be
a good choice, here, for that reason!
Grickle Formulations will vary in composition, depending on how hot the
base-glass is, that it is being applied to, how hot the grickle is, what colors
one wants (if the grickle is a paint), and how much fluxing (or other priming)
action one wants. Grickle may contain
any of the materials listed in the materials “palette” section here above, and
prominently feature borax, water glass, silane compounds, metals, metal oxides,
and fine-grained sand, to include fine-ground glass… And possibly more! See the section (here) titled “Miracles
Happen Here Formulations” for more (speculative to be sure) information.
Depending on what wants to do, and what is possible, grickle
may be thin liquid paint, like a toothpaste, like lipstick, like chalk for your
chalkboard, or like graphite for your mechanical pencil (solid but easily
eroded), or like a pellet for your pellet gun.
So, to keep things intuitive, I’ll write of grickle paint,
grickle-paste, grickle-lipstick, grickle pencil sticks, and grickle pellets. For a specific use and color, I don’t know
what form the grickle will take. For one
thing, I can’t find much if anything about the mechanical strength of solid
(anhydrous) borax… But then I thought, even if I knew that parameter, I want to
add other substances to the grickle mix, in an unknown-proportions mix, and no
one will know ahead of time, what the viscosity or mechanical strength of the
grickle-mix will be! So I’ve stopped
obsessing about such things. But we’ve
now expanded our grickle vocabulary!
This will help make sense of the additional “grickle tools” that I’ll now
describe, immediately below, and associated notes about “grickle formulation”
for liquid, paste, stiff gels, pencil lead, etc.
Grickle Paints and Brushes,
Take #2… Of
course, define your need first, formulate your paint accordingly, and THEN
select or design your brush! A grickle
brush may be as previously described under Paint
Brushes for Liquid Glass. If your
grickle paint is a paste, especially a more-viscous (thicker) paste, the
brushes could exist only around the periphery of the applicator-tip, with a
hollow space in the middle, facilitating easy flow. If you want to force-feed the paste through
the handle of the brush, one could use a pump and a motor, OR one could use
peristalsis action! Peristalsis action
would consist of travelling waves of “squeezing” the paste, using pneumatic
actuators or solenoids. Flexible hoses
can only be used if the temperatures are low enough to not damage the hoses,
though. Or use “ice box” technology to
selectively protect your hoses. I see no
reason why solid, actuated “paste squeezers” inside a pipe couldn’t be use to
implement peristalsis. Tight seals
against leaks might be troublesome here, though.
A grickle powder brush (probably with metal bristles) just
MIGHT make sense in SOME applications, but I can’t see it, very well. On a solid (as in glass-wares), the powder
would be most likely to fall off. In
float glass, if the brushes touch the glass, the bristles and powder would
quickly “tar up” with liquid glass. If
one held the brush right above the liquid glass, without contact, and imparted
shocks or vibrations to the brush, causing the powder to fall out, that just
MIGHT work!
Grickle Lipstick Applicator… This item should
speak for itself! Use your (or someone
else’s) mechanical talents to design a suitable high-temperature applicator. If “smearing” of the lipstick is a big
problem, as entire chunks of lipstick break off, placing some stiff (wire?)
bristle-guards around the periphery of the protruding lipstick might help. If one were to use this method to write on a
bottle, pre-annealing, we’d have a message ON a bottle, instead of IN a
bottle! Maybe “Sting” could sing a song
about it, for us!
Grickle Mechanical Pencil… This item, too,
should speak for itself. After we define
our need and then formulate our grickle, we might have added liquid (hydrated)
borax (etc., see “Miracles Happen Here Formulations”
here in this paper), maybe a wee tad of silane
compounds and “water glass”, and, most widely various… Metals, metal oxides,
and-or other coloring agents! Then we
heated and dried the mix (turned the borax into anhydrous borax, etc.)… Since we experimentally determined that this
was the only practical grickle that we could come up with… Oh, I don’t know, all other forms had
“gravity settling”, one-substance-repels-another problems, or other
problems… This is what we came up
with. The “pencil core” here might even
be hollow, with another substance inside of it, or two (or more) solids glued
together side-by-side. In any case, we
now design a grickle mechanical pencil to suit!
Grickle Pen… This might dispense
liquids. It might have a sharp metal
point with a trough on top of it, for digging (plow-style) shallowly into
liquid float glass, or through a layer of just-dumped glass-sands, with liquids
fed into the trough. The liquid here
would usually be a coloring agent, I would think. A grickle pen (for float glass) might also be
a combination as follows: Same as above,
a plow-tipped pen, but no liquids need apply (or be applied). Instead, right above the plow, a powder-laden
brush is VIBRATED to release the powder, without the brush-bristles contacting
the molten glass. This could avoid the
molten-glass “tarring” problem, of course.
Or, liquids and powders could both be applied at the same time, at the
point of a grickle-pen. Tarring of the
plow itself might be a problem. If so,
perhaps a “dry lube” (glass-compatible powder) could be laid down ahead of
time, before grickle-pen contact, for the entire run-length of the pen. The dry lube would prevent tarring, and
hopefully (if properly formulated) melt into the glass, leaving no, or few,
other marks. Fine-ground glass-sand
(plus additives?) might do the trick. If
so, then, of course the sand-glass should be applied while much colder than the
targeted molten glass.
For the above applications of a powder or a
sand, think of it this way: An hourglass with properly formulated sand
dispenses, in a gravity-fed style, sand at a fixed rate. Now, in your mind’s eye, place some
constricting wire bristles right there at the top of the hour-glass
throat. If designed properly, the sand
will NOT flow, except when the hourglass is shocked, shaken, or vibrated (“Shaken,
not stirred”, per Agent 007!). So we
could do the same, in a high-end grickle pen.
Archimedes-screw augurs feed the feedstock (sand) to a gravity-powered
chamber. The gravity-powered chamber has
level sensors to turn the augurs on and off, to keep a constant gravity load (level
of feedstock) imposed on the dispenser-throat.
The entire grickle pen is vibrated only when the product needs to be
dispensed. A suitable name for this
apparatus is a “vibrating grickle pen”,
then. If this tool
spreads the product too far and too wide, a “focusing funnel” may need to be
applied (perhaps selectively, in place, or not in place) between the pen and
the target.
Grickle Gun… This item would be a
mechanical or (probably more likely) pneumatically-powered low-powered gun, for
shooting tiny grickle pellets at glass.
Such pellets would be formulated to be hard and strong. It could perhaps be used artistically on
molten glass, for artistic “splatter” or “explosion” effects (including the use
of colored grickle pellets), but I see few other potential uses with liquid
glass. Where I do see a strong potential
use, is for roughening up glass-wares (bottles, jars, etc.) after formation,
but before annealing. The pellets would
roughen up the glass, AND, perhaps more importantly, embed some metal oxides
(and-or other solid primers or fluxes) into (onto) the surface of the glass, in
preparation for a cover layer of “grickle glass”, which all (base glass,
primer-flux, usually-colored grickle glass) then goes to be annealed. Grickle glass here would usually contain a
lot of LionGlass, I would think, for the low melting point thereof.
Grickle-Blobbing Tool… This item is AKA the
grickle-blobber, and I consider it to be a PRIME tool for grickling,
seriously! It would have very little use
in the float-glass world, beyond strange artistic uses, I would think. In the glass-ware (solid not liquid target)
world, I could see a lot of uses!
We’ve already touched on what is today called a draw-off
syringe. We beef it up here to create
the grickle-blobber, which can draw off (pick up) thin (hot) glass for a
refill, and apply it. It is a
bi-directional syringe, then, just like a pipette with a bulb, but designed for
much higher temperatures, and for automation.
The plunger handle-end is specifically designed with a mechanical
interface to robotic (or other-mechanical) grappling fingers, so that the robot
may pick up, or dispense, liquid glass, and can not only grip the plunger, but
also release it, free to spin. Motors
and cogs (or other methods) allow the robot to spin the body of the blobber,
without spinning the plunger. This reduces
mechanical complexity, of course.
Parenthetically, this tool might also be loaded or re-loaded by a ladle,
as opposed to using a draw-off (pipette-style) suck-off-type action.
Anyone familiar with glass-working will be thoroughly
familiar with just HOW very thick (viscous) glass is, at any sort of sensible
working temperature. A “blob” of glass
will form at the dispensing tip of this draw-off-syringe-like “grickle-blobber”. At the dispensing end of this tool, there
should most likely be an array of quite a few holes, not just one, to get the
molten glass in and out more easily… In
the face of just how thick the molten glass is!
The body of this tool should probably include heating provisions, to
heat the contained grickle, when needed, in a controlled manner. Gas fired is possible, but electrically
heated sounds better to me. Getting
power to the tool (while you spin it, especially) may be troublesome, but could
be solved with flexible, spring-loaded, coiled power wires, wireless power
transfer, carbon electrical contact power brushes, or some combination
thereof. A bit exotically, radio waves
can even be used as a source of wireless power!
Now your robot (remote controlled by a human,
or by AI) is free to spin the blob of glass dangling off of the tip of your
tool, to keep it from falling off. Just
like twirling a table implement to keep the honey from dripping off, you see. It is free to enlarge or contract the
blob. And, of course, it can lower the
blob to contact the target, dispensing glass on contact. Raise the blob to stop dispensing. Add scissors and-or a “slop catcher”,
perhaps, between the tool and the target, that can be engaged when no dripping
is desired, and removed when dispensing.
It can reload on (usually colored) glass supplies, from “hot pots” or
crucibles. If multiple colors are
needed, your tool can “spit out” the excess of one color of molten glass, back
into the crucible, and pick up another color, from another “hot pot”. Maybe spit out the mixed colors when doing
that, in between the two colors, to a “slag heap” for later recycling us as
scrap glass, AKA “cullet”. Or provide a grickle-blobber for
each color! Parenthetically, the “slop
catcher”, if used, can periodically be dumped to the crucible, or to the slag
heap, as is appropriate.
Now the next variation on the above, I’m not too sure about
it, but will mention it in the name of completeness. The grickle-blobber could carry a load of a
high-melting-point glass, such as borosilicate glass, as a carrier only. This would NOT be the grickle intended for
the target! The stiffer blob of
borosilicate glass could then be dipped into a thinner grickle (especially
LionGlass-based grickle, of course) as the load intended for dispensing to the
target. If the base-blob is large, large
loads of grickle could be carried. If
liquid-grickle pens are used to pre-load a fairly large base-blob of
borosilicate glass, with stripes of several colors of thin grickle, and THEN
the base-blob is spun out onto the target, we could get some very artistic
(“tie-dyed” style) looking results!
Intermixing of glass types (and resulting frequent trips to the slag
heap) might be troublesome (to include the base-of-the-bulb borosilicate glass
getting mixed into the grickle). The
full implications are not all clear to me.
But this tool and method seems to be very possible to me…
Robo-Grickle Scissors Tool… This item is needed
(glass-workers, glass-video viewers, and glass-TV-show-viewers will know) to
cut off the dangling, viscous (thick) glass “drool” of tools like the
grickle-blobber and the hot-glass gun.
Else the “glass drool” will mess everything up!
Grickle-Slobber-Pickup Tool… This item isn’t
needed for the grickle-blobber, which can “twist and turn” and pull back its
own slobber, after it is cut with scissors.
Adding such “twist and turn” capabilities to the unwieldy hot-glass gun
sounds prohibitive to me… And so then we need the grickle-slobber pickup tool,
for that one special case!
Parenthetically at this point, I would add, it MIGHT be possible to
shrink down the hot-glass gun in size, to the point that it can be used for
fine art, or touch-up jobs, rather than “broadcast-spreading” a large layer of
glass, and therefor deserve to sometimes be called a “grickle tool” in its
smaller form, but somehow I doubt that this is practical… But that’s just me!
As is appropriate, the grickle-slobber pickup tool may dump
its gathered glass-slobber back into a crucible, or onto a “slag heap”.
Laser Grickle Tool… This item can be used
(along with video-camera-based visual inspection, monitored by humans and-or by
AI) to spot bubbles in the glass, which might be by-products of various
artistic measures and additives. When
spotted, a bubble may have some laser-heat targeted right above it, to “thin
out” the thick liquid glass in that spot.
Then a short, sharp blast of the laser can punch a glass-hole, and
“burst your glass bubble”, letting the bubble-gasses escape. This might leave a pock-mark, which may be
tolerable. Pock-marks (laser marks)
could be deliberately added for artistic effects, if desired. Other laser uses may include who-knows-what,
but might include, for example, adding excessive heat to a spot for “spot
welding” of otherwise-incompatible (embedded?) materials. Suppose we want to add 33 COE (borosilicate)
colored glass-sand to 90 COE base glass, because we feel we just HAVE to have a
certain bright color that we can’t get otherwise, AND the ribbon-flow will
otherwise never reach the required high-enough heat to accommodate this. A special laser treatment to selectively
over-heat this area might be needed. I
don’t know if we can EVER (by adding foreign materials to add more materials
flexibility for “compliance”. perhaps?) mix 33 COE and 90 COE glass this way,
but it just MIGHT be possible, and PART of the “fix” might involve the use of
lasers.
Generically, all of the above SIMPLE tools (excluding the
hot-glass gun and the laser) could be called “molten glass applicators”, or
dispensers, or dispenser-applicators! Or
lump them all together and call them “grickle tools”! Suit yourself! (One can also just dump molten glass out of a
ladle, or drip it [as a VERY thick liquid] off of a honey-ladle-style globe-shape
[bulb] with a handle on it, but those kinds of things are very, very well known
in glass-working circles already).
Applying Hot Glass to Hot Glassware
As
already mentioned, LionGlass (with a much-lower melting point compared to classical
glass, which should NOT be confused with Classical Gas, see https://en.wikipedia.org/wiki/Classical_Gas and https://www.youtube.com/watch?v=mREi_Bb85Sk )
could be applied as a “grickle” surface treatment to classical forms of
glass. I personally have OFTEN been
annoyed by cheap glass items (drinking glasses, humming-bird feeders, etc.)
where glass has been painted with acrylic or epoxy-based (or other?)
paints. Even if you sandblast the areas
of the glass to be painted (which will cost extra money), before applying
paint, give your painted glass some time in the sun and rain, or in the
dishwasher, and the paint will wear off.
What if, instead, we painted the older (higher-melting-point) glass with
molten LionGlass (or a blend of LionGlass, coloring agents, and additives)
instead? I highly suspect that such a
method could provide results more durable than paint on glass. This (LionGlass applied to other forms of
glassware) is ONE of the reasons why I (above) described molten-glass applicator
tools. Note also in passing that it
might be possible to apply LionGlass to cooling-down ceramics, for interesting
results. However, since MANY types and
colors of glaze are already commonly used on ceramics, applying LionGlass here
may not be of much added value.
For forming glass bottles and
other kinds of glassware, ask The Google, Which Knows All Things! Here is ONE basic link that I found
easily: https://www.oberk.com/packaging-crash-course/glass-bottle-formation says “Regardless of the process used, once the bottle has
been completely formed, it is removed from the mold and transferred to the
annealing lehr. The lehr reheats the bottes to a temperature of about 1,050
degrees Fahrenheit then gradually cools them to about 390F.“ 1,050 F is 565 C. Now the following is a bit speculative, but
we (far above) have gone over the temperature-v/s-viscosity graph (or crudely
put, the “melting point”) of LionGlass.
It should be possible to paint hot LionGlass (plus additives, especially
coloring agents) onto the (harder, higher-melting-point) hot bottles or other
glassware. If the LionGlass “paint” is
kept thin, applying this hotter paint to a cooler glassware piece will
hopefully not crack the glassware from thermal shock. If such cracking IS a problem, then partially
re-heat the glassware up towards the desired 565 C (base glass) annealing
point, take it back out of the kiln, and THEN apply the hot-glass paint, so
that the temperature difference between the two (base glass and paint-glass)
isn’t so large.
There may be balancing acts in play,
here. Apply the paint-glass to a colder
glass-piece risks cracking from thermal shock, but it reduces processing steps
(is more affordable), and the excess heat of the glass-paint will quickly be
heat-sunk into the base glass. That is,
the hot glass-paint should quickly cool down (and solidify), reducing the risks
of having the hot-glass paint “running” in response to gravity. Partly re-heating the glass vessel before
applying hot-glass “paint” reduces the risks of cracking the glass-piece (from
thermal shock), and may yield a better-bonded (“fused”) glass-to-glass bond,
but increases the risks of “paint running”, and will most likely increase costs,
especially if the work is done by humans.
The proposed grickle-blobbing tool may perhaps work well here for this
application, for applying the grickle-paint.
However, it might NOT work so well, if we are highly dependent on rapid
cooling of the applied grickle paint, and the grickle-blob is large. This is because briefly, on contact with the
base glass, the entire grickle-blob will be shedding heat into the base-glass,
if my hopefully-sensible assumptions are all correct.
Placing the glass-ware pieces tilted
against gravity (better yet, flat) with respect to the glass-painted area
during the annealing process would also help prevent “running” of the
painted-on “grickle” glass layer.
At this point, I should probably (in
the name of completeness) add comments that glass-workers (and people like me,
who have watched glassblowing on television!) will be quite familiar with. For low-volume artistic products, humans can
take the workpieces out of a kiln (or off of the glassblowing tool), and work
the piece on a hot (or high-temperature-tolerant) work bench. Then after the human-added work, put the
artistic piece back in the “lehr”, for annealing and slow cooling. And during that move, hope not to drop the
piece because of the high heat exposure!
For higher-volume (more affordable) products, it might be best to keep
the product in a kiln (or at least, in general, a hot environment), and do the
work via automated tools, or robots. The
simpler tools (described here above) should certainly be amenable to direct
human use. A full-featured hot-glass gun
may be too large, too hot, and-or too unwieldy for direct human uses, but I
could be wrong.
Now, for achieving a better (fused)
bond between your painted (or otherwise applied) layer of “hot-glass paint”
(AKA grickle) onto your base-glass piece, you may want to apply some chemical
assistance, as a primer-layer, or into the glass-layers being fused together
(base layer plus molten-glass “paint”), or both base plus “grickle” paint. Sand-blasting is possible too, but I think
that is too messy and expensive for this application. The chemistry involved here is “over my head”
in terms of me making optimal suggestions, so I will just “wing it” with what
I’ve been able to find, in general terms.
I’ll not clutter this up with many links… “Google” it for yourself, especially since
this paper may be getting too long already!
However, below is what I think that I have learned!
Metal oxides (or more exotically, metal fluorides) make good
“fluxes” for reducing the melting point of the base glass, adding
inter-molecular bonds, and sometimes, other benefits. Metals (for these oxides or fluorides)
include lead, boron, lithium, and potassium. These are the most commonly used (generally
the best). Sometimes also listed for
this are sodium, magnesium, calcium, zinc, and barium; these are less
effective, and often cause the glass to become cloudy over time.
Now metal oxides are generally solids, and can be applied as
a powder. Power can be applied with
brushes, but that is messy, and the powder can fall off of the “painted”
object. This is why the further-above
“grickle gun” was described. If using a
grickle gun with grickle pellets, be sure to do this in a shielded area,
protecting surrounding areas from flying fragments, and making provisions for
gathering the grickle-pellet fragments for recycling. We would be wise to add the powders (such as
metal oxides) to a liquid, for painting purposes (we’re applying a “primer” grickle
here, before applying the hot-glass “paint”, in case you’ve lost track). From research much further above (see the
“glass-associated materials” section), I think that prime candidates for such
liquids are (or might contain) borax, boric acid, alkysilanes, or sodium
silicate. Alkysilanes in liquid form, for paint? https://en.wikipedia.org/wiki/Silane says “Silanes
are commonly used to apply coatings to surfaces or as an adhesion promoter”, and
“Above 420 °C, silane decomposes into silicon and hydrogen…”
The first quote looks like we could use alkysilanes here, but the second (silane referring to a gas,
not alkysilanes) looks a bit scary. This
is all speculative… It may or may not
work for us. More research on my part
wasn’t very fruitful, partly because I don’t want to pay for papers which may
or may not tell me what I need to know.
If I want to apply some form of liquid silane compound as a primer, on
hot glass, how hot can the hot glass get, before it won’t work? How cool do I have to keep the silane-compound-containing
liquid paint-primer, before it over-heats while I’m trying to apply it? If any reader cares to chase this down
further, start with a “pay walled” source here, perhaps… https://pubs.acs.org/doi/10.1021/ja01111a043 “Synthesis
and Properties of Some Alkylsilanes”.
But please do search here in this document for “silane” first, because I
have more details in the ”palette” section here.
Another candidate liquid is sodium silicate in water.
This, however, outgasses when heated, which isn’t good for us here. This again is speculative, but is perhaps a
better choice than silane compounds.
Probably your BEST bet, though, is borax! Borax in water for liquid grickle, or
anhydrous borax in grickle pellets, should work. See “borax” details elsewhere in this
document… Along with calcium bicarbonate and hydrates of
sodium carbonate. I have little
else to add, with any confidence.
Other ingredients of the “primer” paint might include some
“grit” in the form of diatomaceous earth, perlite (probably in its
already-expanded form, but perhaps pre-expanded), bits of anhydrous borax, boric
acid, pumice, silica gel, or aluminum silicate. I am still speculating wildly (perhaps you
could already tell!). If adding “gritty”
ingredients is wise here, it is probably only in small amounts.
If the “primer” is a liquid, the liquid won’t tolerate a very
hot (kiln) environment. An open-topped
refrigerated bowl or “ice box” will be a good idea for holding a liquid primer,
AKA “grickle”. Search this document for
“ice box” for details on that. Now your
human worker, machine, or robot can reload his-her-its brushes from a “cool”
bowl! As previously remarked,
hollow-handled brushes can also be designed to dispense force-fed fluids.
The glassware may need to sit “at heat” (or be further
heated) between primer application and paint-glass application, to drive water
out of the primer. If water is used, in
the primer, this seems to be a “sure bet” to me!
After all of that song and dance about applying a “primer”
layer, let me remind you, it may be optional!
If we DO use a primer, we want to “paint” the base glass afterwards (or
apply by some other method) with hot molten LionGlass, or with a
LionGlass-based mix. Also note that, if
the “thin” LionGlass isn’t viscous enough, we could thicken it up with small
bits of ceramic paper (probably not a good choice with such paper as it is
formulated today), ceramic fibers, carbon fibers, glass fibers, rockwool fibers
(perhaps with silanization added to the fibers), or ceramic-gaskets materials. Or apply such thickening agents immediately
after the liquid LionGlass-based mix, as a surface treatment. “Thickening” the LionGlass paint here, may be
needed to prevent it from “running”. See
further above here, and search for “ceramic paper” for details or sources of
such materials. Such materials may also
supply internal slop or “compliance” to guard against different CTEs (thermal
expansion characteristics) of the different types of glass. Adding bits of diatomaceous earth, already-expanded
(or being-expanded-as-it-is-applied) perlite, pumice, silica gel, and-or exotic
materials here (into the hot LionGlass “paint”) may also make sense for the
exact same “compliance” reasons, and-or for other reasons. Or we could simply tolerate a bit of “surface
crackling” when the COE (Coefficient Of Expansion) of
the base-layer glass and “painted onto” layer of glass mismatch each other.
Under the “tools” section of this document, a tool called a
“grickle-blobber” is described. It might
be a useful tool for applying hot LionGlass “paint” to solid (but hot)
glassware, here. I think that, of all
the “grickle” tools described here, this will be your best bet for this
application.
Ceramic paper could perhaps (with appropriate cut-outs of
course) be used as stencils for applying “primer”, primer plus hot molten
glass, or hot molten glass alone. Remove
the stencil at any appropriate step in the process.
With all of the (often irreducible?) complexities described
above, glass-on-glass decorations (or labels) sound like high-dollar features
to me, and unlikely to be applied to common (disposable) bottles for drinks and
for foods.
Glass-on-glass would be suitable for hummingbird feeders, drinking
vessels, carafes, serving bowls, and artworks, for examples. Only in our wildest dream could we use glass-on-glass,
on bottles and jars, to place fine-print (but legible) lists of ingredients and
nutrients! Please prove me wrong!
One the other hand, speculatively, maybe a few very simple
marks (think of branding marks such as the “Nike Swoosh”) could be imparted
affordably to disposable pieces of glassware by automated (robot-wielded) tools
such as the “grickle lipstick applicator” tool or the “grickle mechanical
pencil”. That is, IF the grickle media
(lipstick or pencil-core form) can be formulated (especially to impart color)
to hot glassware, at the pre-annealing stage.
It just MIGHT be possible!
LionGlass and Float-glass
Float-glass
resides in a world significantly different from glassware (bottles and jars,
etc.). Many of my previous remarks here
still apply, but since we’re in a flat environment, “gravity is our friend”
now, a molten-glass layer won’t run off due to gravity, and taking pieces out (of
a kiln) for human hand-working becomes far less plausible here. We’re in an automated environment now, pretty
firmly. Fluxes (or “primer-paints” as
described above) between layers of fused glass may make a lot less sense here, not
only because “gravity flow” of an added layer is no longer a concern, but also because
we presumably can easily afford higher annealing temperatures and longer annealing
and cooling-down timeframes, in a large-scale automated float-glass environment.
I hope that I’m not being too terribly disorganized here, but
I’ll just start spitting out ideas associated with LionGlass, floated glass,
and other possibilities whereby some forms of glass could replace ceramic
tiles. Currently-existing flat glass (glass
panes) and ceramic tiles are fairly cost competitive with each other,
especially at the lower-costs end (says my research, but this paper is already
getting long, so do your own research, please).
However, ceramics are generally fired at much higher temperatures
(except for raku-pottery-style ceramics, which isn’t suitable for use, alone,
as floor tiles, but can be used as wall tiles) than glass, so glass tiles
(especially at yet-lower temperatures as facilitated by LionGlass) may drop
costs even more, and help reduce carbon emissions (through reduced heating
costs).
Three-layered crackle glass (as described above, here,
associated with https://expresstoughening.com/latest-articles/what-are-the-benefits-of-using-crackle-glass/#:~:text=Thanks%20to%20its%20build%2C%20crackle,glass%20in%20a%20busy%20environment ) could perhaps be created using LionGlass (or any other
suitable type of glass, balancing costs and other considerations), and “floated
out” (called a “ribbon” of floated glass) onto the tin bath, or other molten-metal
bath, forming a layer of molten glass.
This is now our base layer. Onto
the top of this freshly-poured base layer of glass, after it travels a small
distance down the lehr-bath, we drop onto the top of it, sheets or panes of
much-colder solid glass (of any suitable glass-type). This glass will crack from thermal
shock. Next, onto the top of the cracked
glass, we dispense (by any suitable means, such as automated hot-glass guns) a
layer of hot suitable glass, especially LionGlass (because it has a low melting
point). The cracked-glass layer won’t
allow much (if any) LionGlass to penetrate far downwards, and, if materials and
handling choices are all formulated appropriately, the glass, broken-glass, glass
3-layer sandwich can travel down the lehr-bath further, for annealing, cooling,
being pulled off, and being cut and diced in the conventional manner. Optimized glass densities and thermal
expansion coefficients, as well as optimal temperatures, should make this process
work. For one thing, even if the
broken-glass layer is slightly less dense than the covering LionGlass layer,
the thick viscosity of a never-overheated LionGlass layer should prevent the
broken-glass shards from rising to the top.
Similarly, the viscosity of the lowest layer of glass (even if less
dense that the broken-glass layer) will be way too thick to sneak upwards through
tiny cracks in the broken-glass layer. Allakhazam,
I give you hopefully-affordable 3-layer crackle glass!
Upon further thought, we may not be “home free” with the
above approach, especially if we’re concerned about the “safety glass” aspects
of crackle glass. I’m really not sure
how exactly it works. Will our middle
cracked layer fuse back together too much during the annealing process, and
will that hurt any of our objectives? If
so, we might need to add a little bit more to our process, as follows: After
the middle layer cracks, force the fragments apart just a wee tad… Induce some small waves onto the liquid tin
bath, and be sure to leave (maybe 2 inches on each side of the ribbon-limits)
some spare room for the fragments to drift apart from one another. Don’t make the cracked middle layer too wide,
that is! And if the induced “storms at
sea” in our tin bath don’t work well, maybe add some close-in blasts of gas, or
add light touches with brushes or other tools.
Adding separation distance may be enough for us, perhaps.
Or it might not be enough!
Maybe we need to add some fine-grain sand or other material to prop the
cracks open, in a manner similar to injecting sand into cracks in the rock, in
oil-and-gas fracking. Use brushes and-or
gas-blasts and-or tin-bath waves to add some mix of the usual suspects for
“grit” into these cracks… Ground-up
anhydrous borax, diatomaceous earth, perlite (probably in its already-expanded
form, but perhaps pre-expanded), pumice, silica
gel, or aluminum silicate are some of the usual suspects here, along with
finely ground glass. Finely ground
COLORED glass might look good here! It
would be best to lightly brush the grit off of the tops of the glass fragments,
I think. Brush them towards the margins
of the ribbon, where the resulting glass will be trimmed off to become “cullet”
for recycling, anyway.
See “embedded wires or mesh” or “Georgian wired glass” here
in this paper for more details, but we could easily ALSO add wire mesh to the
above-described crackle glass, perhaps, for additional safety. After the base layer plus cracked layer is
all finished up and ready to go, sitting there on your tin bath, apply wire
mesh on top of these two layers. And
only after THAT, add your third layer of glass to be annealed, on top (with the
top layer perhaps containing LionGlass).
Then, of course, anneal, cool, and process as usual.
Not to insult your intelligence, Dear Reader, but to make
sure to state the obvious, at one point, at least… All these monkeyshines to
be carried out over the top of the hot tin bath? To include more ideas to follow? No, no humans should be assigned to these
jobs! Too much heat in there, and it is
a nitrogen-hydrogen environment! All of
the tasks should be performed by machines or robots! Smaller tools (grickle tools) can scoot back
and forth, mounted on rails across the bath, with these rails (“rails on
rails”!) mounted on rails themselves, travelling back and forth along the
length of the bath. Think of X-Y
pen-and-paper plotting or printing machines, for example. The cross-bath glass-decorating (or other
processing or glass-pane-adding) movements of robots or machines should be
called the “Y” dimension, and alongside-the-bath movements should be called the
“X” dimension. In the “X” dimension, the
tools-on-rails-on-rails movement need merely pause or stop, to back-track in
the “X” dimension, with respect to the constantly-flowing glass-ribbon flowing
underneath the tools.
I never promised you a
glass-rose garden! Yes, by now it should be starting to become
clear that many ideas that I propose here won’t come to fruition easily or quickly. Float-glass machines operate constantly, for
many years. It would be prohibitively
expensive to slow one down, or stop it, for the purposes here described. But as Einstein told us, speed is
relative! Keep your tools and
adding-materials operation up with the flow-rate of the glass ribbon, in the
“X” direction, and maybe it will work!
However, what do you do when the tools reach their maximum travel
distance in the “X” direction? One has
to either A) pick up the tools and their support, and move them back to “X
minimum”, to re-start their journey, skipping your adding-materials-to-the-glass-ribbon
activities (producing plain glass for the interim), or B) Do the exact same
thing, just scooting the tools (and their supports) backwards along the rails
at the maximum safe speed that can be attained.
Maybe even C), keep spare tools and tool-beds of each kind, and drop
them in at “X minimum” and pull them off at “X maximum”, leaving no glass-coverage
gaps. “A” sounds absurd! Go with “B”!
“C” sounds expensive, and likely to lengthen your already-long glass
factory, which typically is about ½ kilometer long. If you don’t mind the extra expense of a
longer line, maybe some sort of “D” choice might be, each set of specialized
tools (for specialized purposes) spans 1.5 or 2 times the “X” dimension that
you’d normally expect for it to take up.
Now your specialized tools have more “scoot room” to scoot back and
forth, for a safety margin, or for new versions of a process or glass
product. This would also cut down on (or
eliminate) the need for clear-glass-in-the-interim areas, to allow X-dimension
tool-scooting time. Trade tool-time
flexibility for extra space and factory-length expenses, that is.
In any case, it’s probably clear that the add-on activities
described here (adding layers and-or decorations to the ribbon as it flows)
will require wider, longer, and most likely taller float-glass factories, which
cost $150 to $200 million today. It is
possible, but prohibitively expensive, to “grow” float-glass factories in size,
especially since much (most?) of the added volume will still need to be in a
nitrogen and hydrogen atmosphere. I’ll
keep right on describing my ideas anyway!
Back to three-layered crackle
glass…
An alternative to adding the top-most layer in a molten form
(dispensed by an array of hot-glass guns) is to add glass-sand via an array of “vibrating
grickle pens”, or other method of evenly adding a layer of glass-sand. Applying the top layer of glass as a hot
liquid may splash and splatter, and disturb the underlying cracked glass too
much, but it will require less heat to get this top layer molten and smooth,
before annealing. The top layer being
glass-sand will be vice versa… Applying
the sand could be a more gentle operation, but will require more heat before
annealing. More heat from above can be supplied
by (for examples) propane torches, or (probably a much better idea)
electrically powered heater elements.
The latter, here, won’t pollute your nitrogen-hydrogen environment.
Yet
another alternative is to lower an entire sheet of solid glass onto this
topmost layer, and let it “slump” in the heat.
If this glass is too thin, it won’t be able to support its own weight
(it won’t be strong enough to do that).
If it is heavy, it is expensive, and the product will be heavy. This is if it is supported only around the
edges (by mechanical grippers), as it is lowered. It COULD be lowered down by suction (vacuum)
grippers by an array of such grippers, spread across the whole large pane of
glass. Release the vacuum, and the glass
is released. This array-of-suction-cups
method would distribute the loading of the glass plate’s weight, you see. Formulating heat-tolerant yet effective
suction grippers? Good luck! It just MIGHT be possible, though!
Well,
please excuse my skepticism!
High-temperature-tolerant suction grippers are already here! See the following links: https://www.schmalz.com/en-us/vacuum-technology-for-automation/vacuum-components/vacuum-suction-cups/suction-cups-for-high-temperature-applications/ and https://anver.com/vacuum-components/vacuum-cups/hi_temp_glass_cups/ and https://www.piab.com/en-us/suction-cups-and-soft-grippers/ and
https://www.universalpowerconversion.com/high-temperature-c-1_39_41_546.html#1 and https://pneuforce.com/high-temp
! Please note that these suction cups
may not be quite totally up to speed for the high heat that we have to deal
with. SHORT time frames of excessive
heat MIGHT be tolerable, and we MIGHT help by blowing at-least-slightly-cooled
gasses upon these suction cups, during the deployment process, to alleviate potential
associated heat-induced problems. Better
yet, circulate “cooling fluids” (the general term) through metal back-shells to
these vacuum-powered sucker-cups. I
hereby dub this scheme to be the “octopus glass-grabber”, for obvious reasons.
Yet
ANOTHER alternative is to lower the topmost layer of solid glass in smaller
sections, gripping them by their edges, and avoiding the breaking-under-their-own-weight
problem by simply making them smaller.
Deposit many of them. Segment
them across the ribbon (several discontinuities across the ribbon) rather than
along the length of the ribbon, since many-many supporting mechanical fingers,
grippers, or supports can be arrayed along the length of the solid glass,
avoiding the breaking-under-its-own weight problem, with narrow, long strips of
glass. Now the only disadvantage (that I
can see) is that, when the crackled glass product is cut up for use, the long
stripes under the top-layer discontinuities just MIGHT need to be discarded,
depending on various variables (does this top layer fuse edge-to-edge before or
during annealing, for example).
Now I have yet another idea to describe, which results from
asking, “What if we didn’t want any big, fancy hot-glass guns or ladles out
there dispensing molten top-layer glass for the three-layered crackle glass? Thus-dispensed molten glass might splatter
around, re-arrange the targeted fragments of broken middle-layer glass, and
just generally make a mess (as was already mentioned). AND you don’t like any of the above-listed
alternate methods? Couldn’t we come up
with something fairly simple and gentle?”
The answer is (possibly) YES! See
below…
The Large Thumped-Brush Sand
Dispenser Method works (or could perhaps work) as
follows. We build
a stiff-brushed, large brush, probably with wire bristles. It is large enough to span the entire width
of the ribbon, and the bristles are perhaps on the order of only ½ inch
long. More like 1/4th of an
inch, for the specific use being discussed right now. Arbitrarily, we will call the brush-back side
“down”, and the bristle side “up”. Then
we load the entire brush (while cold and held “upright”) with powdered
LionGlass (plus perhaps some additives).
We make sure that the LionGlass sand or powder (perhaps even raw
LionGlass ingredients, thoroughly mixed, or maybe some other form of
glass-sand, or raw ingredients thereof) is even distributed across the brush, settled
in perhaps by vibrating the whole brush, and-or, with other brushes. Brush the loaded brush!
Next… This may be optional, if the sand loaded into
the brush is made to be a bit “sticky” somehow, but I do recommend the
following step… A THIN retaining layer of formulated liquid (perhaps mostly hydrated
borax and a tiny bit of “water glass”, maybe boric acid, and maybe, but likely
not, some silane compounds… Is applied (likely sprayed) onto the loaded
brush. The sealant may even some contain
tiny fibers of some suitable type(s)… Glass, ceramic, rock-wool, or carbon
fibers, for added strength when dried out.. The whole brush-plus-contents is then heated
and dried. The formulated thin “sealant”
layer will help prevent our sand-load from falling out, before we want it to
fall out. Be sure to COE-match your
“sealant” layer, here, to the glass-type that you’re working with! See notes under “borax” in the “materials
palette” section! Also see calcium bicarbonate and hydrates of sodium
carbonate.
The next
step is an optional test step. Turn the
loaded, dried brush upside down (bristles down), and give it a few VERY GENTLE
test thumps. If nothing falls out, you’re
good to go! If patches of content fall out, turn the brush right-side-up, patch the voids, and
repeat the test process.
The next step is to CAREFULLY (gently) maneuver the
upside-down loaded giant brush out over the hot tin bath, over the top of the
first two layers of glass, and make SURE the brush is exactly where it
belongs. Leave a few fractions of an
inch between the brush and the top of the target. Now activate a bunch of mechanized hammers
(or vibration-inducing rotating mechanisms with off-center masses), and THUMP-THUMP-THUMP
the heck out of the rear of the brush!
All of the sand “load” should fall out.
If not, deploy robotic sand-dispensing vibrating grickle tools (with selectively-timed
vibrating sand-dispensing) to repair the voids in the top layer of glass-sand. Search this document for “vibrating grickle pen” for a more detailed description of just the
right tool for this particular job. Heat
and anneal the glass as normal, or mostly as normal. Depending on what is in that top layer of
glass-sand, the fusing temperature of the glass-sand may be higher than the
normal glass-annealing temperature, in this float-glass process. That will mean, add extra heat, before
annealing! The borax (and-or other
compounds) that was most likely needed here as a precaution again the sand
prematurely falling out? Borax is a
borosilicate-glass ingredient, which fuses at a higher temperature than most
other glasses. So, if very much borax is
needed here, that will provide an additional reason why more heat needs to be
added, after the sand-dumping operation.
(Gently blowing) propane torches from above, or electrically-generated
heat, should do the trick. When done
with all of this, clean your giant brush for the next cycle, of course.
Note that the (anhydrous by the time it is applied) borax in
the protective (sand-retaining) layer will end up sandwiched between the
crackled layer and the top layer of glass.
This is likely to be a good thing, since borax can act as a fusing
agent, or flux. If this isn’t sufficient
here for this particular need, the sand-sealant layer on the loaded brush may
need some other ingredients added to it.
Maybe some metal oxides or fluorides.
Perhaps additives such as water-glass, calcium
bicarbonate, hydrates of sodium carbonate, various
fibers, and-or silane compounds, but these latter ones (I think!) are more-so
associated with other needs or objectives.
OK, a few more added thoughts here: This giant sand-loaded brush is an important
tool, and we’ll use it again (just a wee tad further below, here) for
colored-images sand-painting, possibly with TWO layers of sand (color layer
plus protective clear layer). Or maybe just a colored layer. In any case, we need some good, memorable,
intuitive terminology here! The
sand-load in the brush, and inverted and thumped down onto the molten-glass
ribbon is hereby named the “sand-apple upside-down cake”! And the sand-retaining layer (specially
formulated) is the icing on the cake!
So then my other thoughts to be added here are about the formulated
icing. The above formulation (icing
applied wet) is perhaps workable, but I think that we could do better. Icing applied wet requires more water, which
has to be driven right back out, compared to the following: Apply your icing in the form of finely ground
anhydrous borax (plus other dry ingredients, such as calcium carbonate and
sodium carbonate). Next, apply
(presumably small) amounts of tiny and small-diameter ceramic, glass, carbon,
or rockwool fibers, if this idea is wise.
Fibers should be applied dry as well.
Some or all of the fibers (if used here at all) might possibly be
“silanized”, in a manner similar to what was described here: https://onlinelibrary.wiley.com/doi/10.1002/anie.202308822 (this is a repeated
link in this paper).
Anyway, it might be best to apply your icing dry, and then
spray or mist it with water, before heating it to re-solidify the powdered
borax into a thin solid sand-retaining “icing” layer. This conserves water and therefor also
drying-and-curing heat.
Selecting the (large brush for sand-apple upside-down cake) brush-bristles
very carefully, here, is paramount. The
brushes will need to withstand the heat of curing (drying) the water out of the
borax icing, and brief exposure to more heat during the sand-dumping operation. The bristles will also need to cling to the cake
“icing” long enough to retain the sand, but NOT cling so tightly as to never
release the sand, when the brush is “thumped”.
So adhesion to the bristles (as well as icing thickness and strength)
needs to be carefully adjusted. Bristles spacing matters, as well. This whole idea could be made to work… Or not!
I’d like to see someone try it, at least, for sure!
If it can’t be made to work, what other choices are
there? Excessively strong icing, and
dump-able bristles, that can be molten into the glass? Such bristles would somehow need to be
released from the brush-back, at the correct time. On this one, I am frankly in too deep for me!
I don’t want to set myself up as THE grickle-grammar-and-usage
NAZI, but I don’t think that the giant sand-loaded get-thumped-upside-down
brush should be called a grickle tool.
This brush is a VERY broad brush tool, for dispensing an entire layer of
glass-sand, and, in my mind at least, grickle tools are smaller tools, for
more-selective applications. The
“grickle” term isn’t for any kind of tool that applies (or can apply) an entire layer of
glass or glass-sand, that is.
For Creating Colored-Images Float
Glass, we could be creating “sand paintings” on glass, or “fake
stained glass” with real colored-glass fragments (but with the metal solder,
usually mostly lead, between the glass pieces, replaced by dark glass), or art
glass with “misc.” inclusions or additives.
Or with small artistic “cookies” added on top of the
glass (or at least partially slumped or fused in, sinking down into the
base-layer glass), which I will define later (below). Such types of glass (now just listed) could
be used as window glass (in which case we’d usually want to go light on the
colors, more transparent and less opaque), or as floor tiles, or as wall tiles,
and more. With tiles and other artistic
applications, we may not care much at all, about transparent versus opaque. For floor tiles only, we may want to add some
friction, to prevent “slippery when wet”!
For the latter case, apply (at an appropriate spot in the melting-and-annealing
process) some “grit” to the exposed surface.
Again, the usual suspects (for grit) are ground-up
anhydrous borax, diatomaceous earth, perlite (probably in its already-expanded
form, but perhaps pre-expanded), pumice, silica
gel, sand, or aluminum silicate. A
generic comment (about glass tiles) that I have seen is, take SOME precautions
against looking right through the glass, and seeing the floor-or-wall glue (or
grout, mortar) stripes behind the glass, for mounting the tiles! That looks ugly! If the glass tiles are fairly clear,
sandblast them on the rear side, and-or paint them, cover them in a thin layer
of cementitious product, or glue some sort of covering on the rear, before
selling the product. We don’t want to
see rows of tile-glue or grout, through the glass!
For Creating Sand-Painted Float
Glass, as a specific type of image for glass window panes or tiles,
this is a process that (optimally, hopefully) uses the just-now above-described
“large thumped-brush sand dispenser”, so we’ll cover it first. For alternates (to thumping
your large brush) and lots of agonizing over all of that. see further above, under the Three-layered crackle glass heading.
While it
is still fresh in our minds, let’s go over the large-brush thumping method, for
colored images! Here, we do the same
things as were listed above, except the giant brush-bristles will perhaps be
longer (maybe ½ inch total) to carry TWO layers of sand (plus additives maybe).
The bottom
layer (when loading the brush; the TOP layer after being applied to the base
glass) will simply be a clear-glass formulation (perhaps mostly LionGlass), to
protect the colored-glass middle layer.
It is possible, actually, that this layer isn’t needed, in which
case… Skip it! And use shorter bristles on the brush, for
the colored-glass-sand surface layer only!!
As I
understand it, certain colors (green and brown, for examples) are easy to
produce with soda-lime glass, often with metal oxide additives. Other (and often brighter) colors are best
obtained in borosilicate glass, often with metal additives. If using colored glass-sand here, covering
the borosilicate colored-sand layer (in our sand-apple upside-down cake when
inverted and dumped onto the base glass) may be especially important, if we
want to NOT raise the annealing temperate (of the
overall product) very high. In this
case, we can get away with merely “sintering”, or just “partially sintering”
the brighter-colored borosilicate glasses, in our palette, that end up in the
middle of our final 3-layered glass, and the top or cap-off layer (LionGlass
based for low melting point?) will keep the partially sintered (or even still
just remaining in the glass-sand state) encapsulated, so that it won’t fall out
of our “sand-painting glass”, or “fake stained glass”. COE (Coefficient Of
Expansion) and associated cracking concerns may entirely prohibit mixing
glass-types here, though, as I have just proposed above. Find a fix if it is possible to do so,
please!
The
“icing” on our sand-apple upside-down cake? The specially formulated
layer, to keep the sands from falling out of our large to-be-thumped brush? It may not be strong enough to contain TWO
layers of sand (colored sand plus encapsulating “top” layer of the finished
product). It might just barely be able
to carry ONE layer (the colored layer) at once.
If this is the case, keep the colored layer thin, of course, and apply
that layer all by itself. Apply the
clear (sealing, encapsulating) layer in another application of the large
sand-carrying “thumped brush”… OR apply the clear layer (as sand) with a “vibrating
grickle pen”, or other method or application tool. Suggestion:
Research how agricultural implements apply evenly distributed granular
fertilizers, which resemble sand. Apply
entire sheets of glass, as previously described, for example, is another
option.
Now it is
entirely possible that the whole sand-apple upside-down cake (and large thumped
brush) idea is implausible, or even impossible.
Formulating the bristles and bristle-grabbing “icing” layer to be JUST
right, just strong enough but not too strong, might be too tricky! The R&D costs for this might be
prohibitive! In that case, “drop back
and punt”, and consider the following (further below) alternatives, now that we
are considering the full implications of a coloring layer, unlike the “3-layer
crackled glass” section further up.
Another (probably quite serious) problem with the sand-apple upside-down
cake is that if the “icing” contains too much borax (whatever “too much” may
be), the “COE” (Coefficient Of Expansion) of the
glass, at that layer, will be disturbed.
Now we’ll get cracking, unless we’re using a COE-of-the-base-layer type
of glass plus glass-sand, that will solve this
problem. Or some other solution can be
found! As usual, I’ll just keep right on
speculating wildly! But do please look
at “borax” under the “materials palette” section of this paper here, along with calcium bicarbonate and hydrates of sodium carbonate, etc., before
giving up all hope!
Drop the
thumped-brush (etc.) idea and consider breaking your sand-paintings down to
create smaller, individual paintings, and exclude the idea of GIANT images,
such as ones that straddle the entire width of the ribbon. Paint the sand-paintings (by human hands or
by machines or robots) onto SMALLER solid panes of glass. Use a glue (containing water-glass, and-or
water and borax, etc.) to hold the glass-sand, lest it get jostled off of the
glass during processing and placement.
Formulate your glue to avoid COE-mismatches-the-glass problems, and-or,
apply it to be very thin. Cure (heat,
dry) the glue plus sand. Pre-heat the
(still SOLID!) assemblies (to avoid thermal shock) before dropping them onto
the float glass), using mechanical edge-grippers (“fingers”). IMHO (In My Humble Opinion), a few cracks,
from thermal shock, should be tolerable, especially if most of the cracks
re-fuse together later, or the slightly-defective “seconds” of production runs
can be sold at a profit, anyway. After
placement, optionally, optically inspect the placement results for defects (deep
gripper “finger” marks for example), and repair defects using the “vibrating
grickle pen”. Where one sand-painting
pane meets another, on the ribbon, cut and discard the border areas (this is an
unavoidable price of this method, it seems to me).
Yet
another idea is to use the “octopus glass-grabber”, which allows one to use the
entire width of the ribbon (minus margins) to create very LARGE sand-painting
images. As immediately above, humans,
robots, or other machines lay down different areas of different colors of sand,
on top of glass-compatible glue, and the giant pane of glass (plus glue and
glass-sands). Then the assembly is
heated and cured, pre-heated, dropped onto the ribbon, etc. The one difference here is that one needs to
place glass-grabbing (“octopus”) suckers ahead of time, onto this artwork,
before the sand-painting is applied.
Such sucker-locations should be strategically selected,
and repaired (via vibrating grickle-pen as usual) after placement. Frankly, if I was shelling out the R&D
money, I’d pick this “racehorse at the tracks” before I would select the
large-inverted-brush method! But yes,
after all of this, one would probably be best off, also applying a clear
protective layer of glass, by some method or another… Especially if the applied sand-glass includes
ground-up borosilicate colored glasses, with their higher melting points, and the
ribbon-annealing temperature is set down low.
So what if the sand never thoroughly melts, or even gets sintered, so
long as it is encapsulated, and can’t fall out?
Yet
another alternative for creating large colored-sand art, and then dumping it
onto the ribbon of glass, would be to arrange colored glass-sands onto the top
of a base layer of sand (“sand casting”, look it up), then dump molten glass on
top of it (How would one keep the glass-dumping operation from disturbing the
colored sands pattern? I don’t know.),
and let the liquid glass ooze down into the cracks between the colored sands. Cool the product slowly, remove the colored-sand
art from the junk-sand sand-bed, and clean it up. It sounds messy to me! Perhaps the sand-bed could be replaced by a
mold-box lined with “kiln wash” and-or suitable ceramic paper, that doesn’t
bond to molten glass.
(Back, now, to the sand-apple upside-down cake). The colored-glass-sand layer, in either case
(protected or not protected by a clear-glass layer) is an artistic arrangement
of different colors of glass, crunched down into sand, and then settled into
the giant brush, appropriate colors to appropriate spots. Then (just as previously described in the
case of the crackled glass), it is covered with a thin cover layer of a liquid
specially-formulated solution, heated, dried, inverted, and “thumped” onto the
base layer of glass. And
repaired (if needed, at voids) by selectively-vibrating sand-loaded smaller
grickle tools. In this case,
unlike the previously listed case (3-layer crackled glass), there is no need to
induce waves in the hot-tin bath, nor to apply “grit” in cracks (there are no
cracks), nor to brush the grit around.
Otherwise, this version of sand-painting is much like the case where we
applied a clear-glass top layer to the 3-layer crackle glass. These, then, are two fairly-much-different
uses for the “Large Thumped-Brush Sand Dispenser Method” as described above,
then.
Dear
Reader, I’m sorry for this mess, but… Please bless this mess! However colored-glass sand may be applied,
the following comments may apply! “Vibrating
grickle pen” methods of broad-based applications, or selective-repair
applications, may threaten to blur boundaries between different colors of
sand. Sometimes this is OK. Sometimes it is not, and we want sharp
boundaries! If the latter is the case,
then, between pen and target, we’ll want to deploy a shield or shields. The artist (remote-controlling human, or
robot, or machine) may need straight shields, curved shields, pointed shields,
etc. Or small “templates” or “masks”, if
you will, in addition to sand-focusing “funnels” for sharp lines… All selectively applied or not applied,
between sand-pen and target. “Steer”
your sand to your target, selectively, is the general idea. Add or subtract complexity at will, and be
sure to charge your customers accordingly!
Or donate generously to your customers, giving more than you get in
return… Suit yourself! (I’m not your boss, and don’t really want to
be.)
Another method
of attaining sharp boundaries between different colors of applied glass-sand
may be the “barrier method”. I have used
this myself, in art compositions of different colors of wet concrete, where I
used vinyl or thick (stiff) plastic as the barrier, and yanked the barrier out
rapidly, to tap into the inertia of the separated colors of concrete… Or slowly, while tamping down selectively on
the nearby areas of being-separated colors of concrete. In the glass world, perhaps we’d need to
apply a thin layer of isolating clear (or white) glass-sand to the surface of
the being-decorated molten base glass layer (to prevent “tarring” of the edge
of the barrier), apply the barrier, apply the colored glass-sands, and then
yank out the barrier. Here, the barrier
could be metallic, or any other (hopefully low-friction, non-sticky, non-clingy)
high-temperature-tolerant material. Vibrating the barrier as you remove it, will perhaps alleviate the
sand-clinging problem. In this
case, the applied barrier will need to be tall enough to allow it to be
gripped, and then removed.
The
barriers could also be left embedded in the sand (leave it there to be molten
and-or annealed, and hope that it is dissolved into the glass, and that it mostly
disappears into the glass; such that it won’t be very visible afterwards). It might be composed of the usual
suspects… Glass fibers, rockwool, fine
metal, or carbon fibers (fiber or wire mesh), coated with silane compounds,
borax, and-or “water-glass”, “grit” bulk filler, and-or other substances. In this case, we want this barrier to be
little if any taller than the sand-layer being
dumped. We don’t want the barrier to
leave visible marks or scars (in the final product) as the excessively tall
wall or barrier crumples or topples down in the heat, but we still want it to
separate different colors of sand. We
may want the grickle-tool (or other tool) sand-depositor to dump (perhaps with
the assistance of a sand-flow-focusing funnel) the sand into a heap (or thick ridge-line)
some distance removed from the barrier, and then brush the sand gently towards
the barrier. This would prevent sand
grains from bouncing and “jumping the fence”, hopefully.
Such a
barrier could even be placed into a large “thumped-brush”, in the “sand-apple
upside-down cake” method, for keeping clean lines between the colors. The barrier is then “dumped” along with the
sands-load, and is incorporated into the glass.
A melts-into-the-glass barrier can be compared to “dissolving stitches”
in surgery, for an intuitive understanding.
Or the barrier might NOT melt into the glass, but simply stay
there! Many artists (in 2-dimensional
forms of art) add dark lines between different-colored areas, to highlight the color
boundaries. The same thing MIGHT be done
in flat art-glass, using, say, thin strips of Kovar. Kovar is suitable for borosilicate glass, for
COE (Coefficient Of Expansion) concerns. Other types of metals or alloys might be
formulated to match other “COEs” in other glass-types.
Depending
on what method is used to apply a clear cover layer (if any is applied, over
the colored layer), it may be necessary to anneal (even fuse) the base plus
color layers, then cool them back down, before applying the clear cover
layer... And then re-heating the whole
mess once again! This might be needed to
prevent the cover layer application process from damaging the color layer. Clearly, we want to avoid the extra time,
trouble, and expense, here, if at all possible!
Next,
we’ll want to consider dropping fine-resolution glass (or other) suitable art
(often in “relief” or semi-3-dimensional) onto the top of the molten glass, or
onto the top of molten glass, plus glass-sand.
This is (as previously mentioned) what I will call a
“cookie”.
If the
cookie is applied AFTER the colored sand is applied, the bonding between the
cookie and the base layer will be hampered, or will require more heat for
bonding or fusing. If the cookie is
applied BEFORE the colored sand is applied, it would be wise to brush stray sand
off of the cookie before annealing, and-or, before adding a “sealing” layer of
glass or sand. In any case, adding a
SOLID (not sand) layer of clear sealing glass over the top of a
non-counter-sunk or pushed-in cookie (if the cookie “pops out” too much) might
barely be tolerable if the top layer “slumps” enough to accommodate it. CLEARLY here (sly joke implied), caution is
advised!
“Cookies” and Hobby Notes
Follow… There could be MANY
kinds of “cookies” here, and I’ll try to NOT go on and on too terribly long
here! As implied above, these are
higher-resolution smaller glass feature (focal) pieces of art. We might sand-paint and grickle-paint grass
(plus mountains, sky, clouds, and blue skies in the background), for example,
and then place “cookie” cows or other animals on top of the grass. I’ll occasionally digress
a wee tad, into other hobby and personal-hobby notes, along the way…
After
having researched the glass-hobbies world, I must start out by emphasizing “COE
of the glass-types compatibility”. It
seems that “COE” (Coefficient Of Expansion) is really
the same thing as “CTE” (Coefficient of Thermal Expansion”). The glass-working world uses COE while the
engineering world uses CTE. So now I
have to try to recall to use COE here, and not CTE.
In any
case, mismatching your COE of different glass types very quickly leads to
cracking, instead of nice, smooth glass-to-glass fusing. This problem is so bad that many
glass-workers or hobbyists will stick to only ONE supplier of (for example) COE
90 glass, for fear of one glass-manufacturer’s “COE 90” mismatching that of
another. This problem may invalidate
MUCH of my wild speculations throughout much of the above paper… But I’ll leave my speculations in place, in
case someone will be inspired to somehow fix this problem, in at least a few
cases, or otherwise gain value from the above, in general!
Here’s what
I think that I have learned; Note that “COE” here is with respect to degrees
“C” and not “F”.
120 COE (Lead-Oxide-Containing Satake): Has a low melting
point. Not as commonly used as some other
types.
113 COE (NON-Lead-Oxide-Containing Satake; Soda-Lime only): Not as commonly used as some other types.
108
COE (Schott clear): Used for paperweights, and little else.
104 COE: Used in lampwork and beadmaking, but less available than 96 and
90. Also called effetre glass, formerly called moretti glass.
96 COE: Easy to cut, and available in a wide range of colors and blends. AKA “Spectrum” glass.
90 COE: Very widely used and available in a variety of colors and blends. AKA “Bullseye” glass.
84 to 87 COE range (not very well controlled typically) is
the “COE number” for float glass, or window-pane glass. Colored glass for this isn’t commonly
available.
84 COE: Takes more time and a higher temperature to fully fuse than 96
COE glass, and isn’t all that commonly used or available for glass art.
33 COE (borosilicate): More forgiving of temperature
variances than softer glass, allows complex constructions by joining separate
components, and is excellent for organic flowing shapes. It has a higher melting point than others. ALSO note that borosilicate glass providers
can deliberately widely vary this number away from “33 COE”!
8 to 14 COE estimate for quartz crystals. My wild translated estimate from other
sources says 7.2… “8 to 14” is from https://www.engineeringtoolbox.com/linear-expansion-coefficients-d_95.html
2.8 COE for Ohara E6 low-expansion
borosilicate glass as is used in large telescopes such as the “Magellan”. Source here is https://mirrorlab.arizona.edu/content/faq
0.55 COE
for quartz glass per https://en.wikipedia.org/wiki/Fused_quartz .
Quartz glass (along with other “engineering” glasses) isn’t
even typically listed for a “glass COE number”, but it is ridiculously
expensive with an off-the-charts-high melting point, and so, doesn’t even
matter, in the (especially artistic) glass-working world. For quartz CRYSTALS, I have researched and
“fudged” an estimated number above, through sources, numbers, and calculations
too boring to be repeated here, but thrown in above, anyway. Crystals here are even more complicated,
because apparently COE varies, depending on which axis (with respect to the
crystal) that one measures it in! So
take my back-of-the-envelope estimate with a grain of salt, please… I tried to force-fit the quartz-crystal
number in with the commonly used estimates as used in the glass-working world,
that is. COE (glass-working) v/s CTE
(engineering world) is SUPPOSED to be the same thing, but somehow, is not, at
times!
A good (but different, with different details) alternative
statement of the above is worth taking a look at, with respect to the
glass-working world: See http://www.glasscampus.com/tutorials/pdf/Chosing%20Glass%20COE.pdf .
ALSO see THIS guide to glass-working and “COE”! https://glassblowingforbeginners.com/types-of-float-glass-and-their-different-coefficients-of-expansion/ … And also see https://gofusing.com/blog/how-to-determine-glass-compatibility/ .
LionGlass COE number remains unknown to me, as do so many
other details about LionGlass. This may
“cover my glass” for me, then, in having speculated so wildly, about so many
things, above! In passing, let me say,
setting up a glass-float operation for creating large panes of art-glass, using
techniques that I’ve been speculating about, may require standardizing to ONE
fairly tightly controlled COE. 90 or 96, perhaps? (I
vote for 90). And then glass-sands and
“cookie” art to place on the ribbon (of the exact same COE-type of glass)
should follow that standard.
Standardization goes a long way!
That said, we can also “cheat” and use non-glass materials
(glues) to bond glass to glass (or other materials, such as whatever our
“cookie” is made of) after the base glass has cooled. For industrial-scale operations, see https://en.wikipedia.org/wiki/Laminated_glass for auto windshields, where the following type of glue is used: https://en.wikipedia.org/wiki/Polyvinyl_butyral , where clear glue is used from layer of glass to layer of glass… For hobbyists, plastic hot-melt glue will do
fine! The hot-melt glue is hot, yes, and
you’d think it might shatter cold glass (from thermal shock) that the hot glue
contacts. I personally tried it (for
glass-on-plastic “permanent repairs”; one can’t undo the bonding) on
hummingbird feeders, and it worked fine!
The heat-carrying capacity of the hot glue is low, so it doesn’t hurt
the glass. See https://www.hotmelt.com/blogs/blog/the-best-adhesives-for-bonding-glass for an excellent write-up.
Personally, here’s my words of wisdom for hobby
work with plastic hot-melt glue: It
“runs”, if you try to add bulk plastic melt, to make a weld thicker. When it “runs” before cooling, it looks ugly,
like snot! Take an ice-cube to
speed-cool it down, on the surface, at least, and the deeper layers of the glue
can be left to cool down slowly. That
is, the ice cube will speed-cool AND shape the cooling glue. Push any “runs” that start to form, back to
the base-pool of the hot-melt, using the ice cube. Also throw in bits or strings of compatible
types of plastic, to thicken the glue.
Plastic clothes line works well for that, AND
for “sewing” together a rip, in, for example, a torn plastic garbage can,
before applying the plastic hot-melt.
Experiment, or look up, what materials work well with hot melt glue. Vinyl isn’t a good choice, I have
learned! (OK, end of digression).
In any case, if all else fails, CHEAT and use glue for bonding
“cookies” to cold glass! For hobbyists,
instead of sand-blasting the glass, for a better glue-bond,
roughen the glass using a Dremel tool with a tungsten carbide bit. When my rear-view mirror’s mounting-base
metal “button” lost its grip on my windshield, that’s what I did. In this case, “Superglue” is commonly used.
Fake
Stained Glass and Penrose Tiles… Dear Reader, I’m sorry if
this is disorganized and-or stream-of-consciousness writing here, but… I thought that before we get too far removed
from creating flat panes of art glass on the float-glass process, I should fit
this in. Suppose we’ve standardized on
COE 90 glass for the base-glass and the add-on “cookies”. The “cookies” could simply be pieces of
glass, in the style of classical stained-glass works. Have your robot or machine place bunches of
small pieces of different colors of glass (that compose your picture) out on
the ribbon, leaving gaps (generally as small as possible, but big enough to
mimic the “real” stained-glass art) between glass-pieces. Now sprinkle on dark-colored glass-sand, to
mimic the color of the lead-based solder in the “real thing”, and brush the
sand into the cracks. Add extra heat to
improve glass-to-glass fusing, if needed, then anneal, and process as usual. It will look much like traditional stained
glass, except one side of it is a layer of clear glass.
Alternately, see “Penrose Tiles”. See https://en.wikipedia.org/wiki/Aperiodic_tiling and https://en.wikipedia.org/wiki/Penrose_tiling , for example. Take
your one shape (and its reflection, see https://en.wikipedia.org/wiki/David_Smith_(hobbyist) for that), or your two shapes (Penrose), and give them
different colors (in glass of course).
Have them placed out on the ribbon, just the same as described above
under “fake stained-glass” above, except that the gaps MIGHT not be needed,
between glass pieces. One could even get
REALLY wild and crazy, and do the following, for example: Create a “run” on your float-glass machine,
using two slightly different colors (yellow and orange, for example). Fab up a big supply of
that. Now do another “run” of
blue and green tiles. Take the results,
and cut them into much larger tiles (of the same two Penrose shapes), with one
set of tiles (shapes) being tiled yellow-and-orange and one set of tiles (of a
different shape) being blue-and-green.
Now place THEM out onto the ribbon, next time around! This is “Penrose squared”! Higher numbers of layers may be possible. Every time you do it, the final-product glass
does get thicker and thicker, though, because of the clear-glass backing layers. If used for floor or wall tiles, one could
cut the final glass product into two basic shapes, and glue them to the floor
or wall, and “Penrose” it yet again, for an utter artistic “riot for the
eyeballs”!
Making these types of cuts in the glass will be slow,
tedious, and expensive, though, I’d bet.
Near-impossible, even. Study up on “lapidary glass cutting” and see https://whirlwindwire.com/products/diamond-wire/iamond-wire-for-glass-cutting.html . As far as I can
tell, a decent “glass-cutting jigsaw” doesn’t exist! So for this scheme (of compounding the
layers) to work, without discarding much of your product that can’t be cleanly
cut off, and without taking ENORMOUS troubles to make strange-angled cuts in
the middle of the product, the first (smallest) tiles would have to be cast
(“sand-cast”?) individually. Otherwise,
I believe that single-level “Penrose tile” on glass backing, coming from the
float-glass machine, and then cut SQUARE as the final product, is plausible.
Penrose-squared, or Penrose-on-Penrose, may not be plausible… Unless a
very wealthy person will pay a LOT of money for it!
Concerning the immediately above, I might be wrong. One might be able to leave clear areas
surrounding a pre-planned “patch” of Penrose tiles (on the first layer or first
pass of “Penrose on Penrose”), to set-up for deliberately discarding clear
glass and “trimmings” off of the colored glass “patch”. Periodically, the clear-of-Penrose-tiles
glass should span the entire width of the ribbon, for easy score-and-break cuts
of entire large sections of glass. Then
use this tool here, for still-impeded cutting:
https://www.glasscrafters.com/diamond-laser-5000-portable-wet-band-saw-11310.html?Session_ID=de08bedfdc3a8ba12a9ceb488c23593d . With good planning,
most of your cuts could then be traditional score-and-break cuts, and the use
of the diamonds-embedded-in-the-blade band saw could be minimized, too. Save the trimmings and re-use them, and-or
sell the colored segments to glass-art hobbyists!
A Whirl-Wind Tour of Glass-Art “Cookies”… Returning now to “cookies”
that can be placed on glass and fused, or placed on cold glass with glue… One can create “canes” of colored glass, and
slice them up. Small canes can be bought
at a popular source here: http://www.muranomillefiori.com/ . Large glass canes can
be tediously created by fusing together many-many thin rods; for a spectacular
example of that, see https://weburbanist.com/2014/06/11/loaves-of-art-classical-works-revealed-in-slices-of-glass/ . This link that
shows a “cane” (less commonly, a “loaf”, in this case) of glass, and from
there, “This loaf of colored glass may not look like much from
the outside, but slice into it and you’ll find an incredible recreation of
Leonardo da Vinci’s painting Virgin on the Rocks. Self-taught glass master Loren
Stump has adapted a 4,000-year-old Middle Eastern technique to produce
detailed images that are only seen when the glass ‘cane’ is cut into
cross-sections.” Sliced by a lapidary
cutter; Probably a diamond-studded-wire cutter, in
this case, I would bet. Slices of such
masterpieces command on the order of $5,000 each, so they are not for the faint
of heart!
For small glass
“cookies” (as I have chosen to call them), see examples at https://www.etsy.com/listing/1461856886/90-coe-fused-glass-hummingbirds-fusing?ga_order=most_relevant&ga_search_type=all&ga_view_type=gallery&ga_search_query=fused+glass+sand&ref=sr_gallery-1-12&sts=1&organic_search_click=1 and at https://www.etsy.com/listing/1471325461/90-coe-fused-glass-cardinals-and-birds?ga_order=most_relevant&ga_search_type=all&ga_view_type=gallery&ga_search_query=fused+glass+sand&ref=sr_gallery-1-13&sts=1&organic_search_click=1 , for almost-randomly chosen examples chosen by Yours
Truly. For larger works of art, the ”cookies” that I imagine would be larger. Perhaps sand-cast, or
created by some similar method? I
cannot find examples of such glass art items, sad to say…
And
https://www.etsy.com/listing/1266151268/pink-fused-glass-flower-garden-with?ga_order=most_relevant&ga_search_type=all&ga_view_type=gallery&ga_search_query=fused+glass+sand&ref=sr_gallery-1-18&frs=1&sts=1&organic_search_click=1 shows an image of a garden scene, combining many small
“cookies”. I imagine MUCH larger scenes
like this, but cast onto large panes from a float-glass machine.
Durable and hard “cookies” of any kind can be durably glued onto cold
glass, as previously mentioned. Think of slices of rock, geodes, gems, or petrified wood; metals
(bronze? “etchings”?),
pottery, or enameled items. Use your
imagination and The Google, for yourself!
This paper is getting to be too long!
More-Opaque Flat Glass
Moving towards Ceramic-Style Tiles, Not
Semi-Transparent Glass, now, I want to mention
(before I forget!) wall-tiles and floor-tiles that are NOT even partially
see-through. Many such items already
exist, but has anyone ever thought of the following: See the custom drawings in this paper (far
above) describing hot-glass guns.
Imagine feeding such a gun (close to the nozzle) with a mix of different
colors of sand-glass, and-or, with some perlite, to be expanded at the last
minute, with perlite expanded at 1,600 degrees F (871 degrees C). One might get a mixed-colors, incompletely-mixed,
fractal or psychedelic “tie-dyed” effect, with the (semi-foamed) glass-diluting
perlite making the end product more affordable.
If the end-product is randomly “lumpy”, perhaps so much the better,
artistically-visually, for wall tiles! “Frozen
bursting bubbles” in a frothy surface finish can be treated with clear sealing
products, for sanitary and appearance purposes, but
permit me not to linger too terribly long…
The Google Knows All Things! Well
OK, it’s been many years since I last bought such products, but here’s one: https://www.poolzoom.com/waterfall-stone-sealer-5-gallon.html .
Note that “waterfall sealer” makes a good search-string term to add, to
find suitable, durable (clear!) products, here.
If the result is too weak for floor
tiles, dial back on the use of perlite, and-or add a layer of a fusible COE-compatible
glass, if possible, and don’t forget to add a thin layer of “grit” for
increasing friction, for reducing “slippery when wet” effects! Or forget the “COE compatible” part, and GLUE
on a suitable and cool-looking glass cover layer!
For glass-based substitutes for ceramic floor tile, including
foamed or semi-foamed products using glass-plus-perlite, I wonder what would
prevent the following approach from being used, for lower-cost product? Lay down a
base layer to reduce the costs, but still make the product thicker, to avoid
breakage, during product production, transport, and application. If appropriate, instead of producing the product
and then cutting it, produce the product in square dividing forms, lined with
“kiln wash” or ceramic paper, if such anti-bonding-to-the-mold liners are even needed
at all. Pour a vaguely COE-compatible
layer of glass over the following type of affordable base layers: Raku
pottery-type materials, cullet (recycled broken glass), “other”, or
gravel. Gravel partially wrapped in (or
supplemented by) “compliant” ceramic paper or appropriate fibers (glass fibers,
carbon fibers, ceramic or rock-wool fibers, for examples) might work. Concrete, I think, is “out”, here, for not
being high-temperature tolerant enough. If the base layer cracks a wee tad, during all of this… Then so what, so long as the surface layer of
the overall product (tile) remains intact?
A bit of “spoilage” can be justified, too, in the name of affordability! Or, cracks can be repaired from the
back-side, using glue or cementitious products.
The “hot-glass gun”-enabled method, in the above applications
for creating a glass or semi-glassy (perlite-foamed) version of fake “ceramic”
tile (as elsewhere), might benefit from “focusing” molten-glassy-product using funnels
and-or shields, directing the molten glassy product (perhaps glassy “froth”) onto
an affordable substrate. Search in this
document for “shield” or (better yet) “template”, to zero in on that aspect of
all things glassy-artistic! I am
suggesting these methods for using two or more colors within the same glassy
tile, that is, using two or more hot-glass guns of different colors, and
keeping clean color-dividing lines, if desired.
For “glassy” (glass-like, or containing glass in a mixture)
tiles that aren’t even vaguely see-through (unlike traditional stained glass
for windows), some other options open up.
In addition to glass, coloring agents, and touches of perlite, one could
also feed into a hot-glass gun, sand that (unlike glass-sands) is NOT intended
to be molten down. Note that “engineered
stone” today often contains quartz sand, in an epoxy binder, especially for
kitchen counter-tops. The epoxy binder
causes the product to not be very heat-tolerant, nor
tolerant of outdoor weathering, especially prolonged sun exposure. Perhaps the epoxy binder could be replaced by
a glass binder, if the formulation is correct?
So take your hot-glass gun, and experimentally feed it different
formulations of glass-sands (possibly including LionGlass for its low melting
point), natural sands NOT intended for melting (quartz sand or otherwise),
perlite, various forms of “grit”, bits of heat-tolerant fibers, and so on. SOME formulation just MIGHT create a durable,
heat-tolerant and outdoor-weathering-resistant product! Note that the “compliance” of expanded or
semi-expanded perlite (plus fibers?) should help to guard against
COE-mismatches between the unmolten sand, and the glassy ingredients. Also note, one could
glue on a clear protective “safety glass” type layer… Perhaps even a thin layer of clear LionGlass,
if it is affordable enough!
For your finishing surface layer of “engineered stone” made
out of (or containing) sand, here is a “riot for the eyeballs” natural-stone
target for you to shoot at: Agate! See https://en.wikipedia.org/wiki/Agate ...
For glassy tiles, why not Include Ceramic Paper in the middle of it?
We don’t want to see through it, anyway!
So lay down some affordable base layer, and mostly disregard tight
compatibility of the COE (Coefficient Of Expansion, of
course) with the middle layer and-or with the top layer, if the glassy-tile
composition even has three layers to start with. On top of the base layer, you place a ceramic
paper template (or discontinuous segments of paper). Over the top of the ceramic paper, sprinkle
your glass-sands which are now free to COE-mismatch the base layer. These colored sands won’t bind (fuse) to the
base layer, because of the ceramic paper.
If there’s not very much of this miss-matching sand (you just HAD to
have some bright 33 COE colors in your 90 COE composition), then the ceramic
tile will still “hang together”, even if the (for example) 33 COE glass-sands
merely “sinter” a bit, and never melt or fuse decently. Avoiding the use of 33 COE sands at the
periphery of the tile might be wise, to keep them from falling out! Capping the composition with a clear-glass
layer will also help “keep it tighter”.
If need be, GLUE the top layer on, after cooling down the base layers. In summary, incorporating ceramic paper into
the middle of glass might enable selective “dodging” of COE-mismatch problems.
Pre-Fab Mosaics might be an obvious idea,
but here goes anyway! We’ve already
covered “fake stained glass”; now do something similar with glass used as floor
tile! “Mosaic” tile on the walls isn’t
traditional, but could be done. Floor
mosaics would be more traditional.
Fabbing them up in a float-glass factory could be done, but (to me at
least) sounds like squandering the precious resources of a fully equipped
float-glass factory! If we DID do that,
we MIGHT attempt to use “looks like grout to me” glass in between the mosaic
fragments (as I speculated about using glass for “fake lead-based solder” in
between pieces of “fake stained glass”), but then, that would inevitably “look
funny” compared to the REAL grout (mortar), when we install sections of the
pre-fabbed mosaic on the floor! We can’t
likely manufacture a 20 foot x 20 foot mosaic in one piece, and transport and
install it, of course, so we have to do it in sections, you see, and join the
sections (on the floor) with real grout or mortar, then. So I think it safe to rule out huge sections
of mosaic, cast in solid glass.
So we’d end up with raggedy-edged pre-fabbed mosaic-segments
perhaps on the order of 1 square yard (or meter), to be placed together,
puzzle-style, on the construction site. The mosaic pieces?
Sure, glass, or glass-tile fabbing techniques as described in this paper
should be relevant, there. They would be
then be placed by robots or machines (for costs savings) in mortar, on
fiber-strengthened (likely cementitious) “backer-board”, similar to what lies
behind your shower tiles.
Such mosaic “tech” (or similar “tech”) already exists… See https://artaic.com/custom-mosaic-design-fabrication-process/ as the only one that I’ve found, that I consider worthy of
inclusion here.
Decorated
Concrete Blocks are another seemingly
obvious idea, but I can’t find such things being mass-produced, or even
semi-mass-produced, in the following sense:
Take your standard nominal concrete block 8 x 8 x 16 inches (actually
7.75 x 7.75 x 15.5 inches), and re-size it to remain the same, except (???) 1/4th
to ½ inches (or so) is depth-subtracted all across one nominal 8 x 16 inch
face, to make room for a glued-on art-glass (or ceramic, porcelain, or other)
face. Decorate “faces” as you see
fit! Now it can sit flush with regular,
boring concrete block, to “liven things up” a bit, hopefully at no prohibitive
costs, if you use only a few of them…
OR, use them to “paint” an entire picture! That would get expensive, AND it would
complicate having to keep track of which block goes where… OR one would have to
glue on the art-glass (etc.) at the work site.
While I’m talking about construction blocks, concrete blocks,
etc., let me dump in some links and notes that aren’t very strictly
glass-related. They ARE candidates for
combining with glass, with recycling, efficiency, and caring about the
environment! See https://www.scientificamerican.com/article/concrete-buildings-could-be-turned-into-rechargeable-batteries/ …
Sad to say, from there, “But for now, a square meter of the building material holds
roughly the energy of two AA batteries”.
Also see https://www.byfusion.com/byblock/ concerning recycled plastics being
turned into construction blocks.
Kitchen
Countertops could also (perhaps) be
formulated using primarily glass and natural sands (not glass-sands) plus
(“miracle happens here”) some of the usual lists of suspects for grit, fiber,
coloring agents, and “chemicals” as described throughout this paper. Search for “engineered stone”, especially in
the slightly-above section here, describing glass or glassy tiles. Once again, a bit of expanding-in-the-heat
perlite might provide “compliance”, and guard against COE-mismatch problems. The “magic mix” would be laid down into a
form, by hot-glass guns. The hot mix
would be compressed by high-temperature-tolerant rollers (think of pie-dough
rollers). If no suitable
rollers-materials can be found to prevent clinging (“sticking”) to the “dough”,
perhaps ceramic-paper wrappers around the rollers would help.
Once again, the “big picture” here is to replace today’s
“engineered stone” countertops’ epoxy sand-binder with glass for a binder, in
hopes that the result would be more high-temperature-tolerant than formulations
containing epoxy. Such glass-based
“engineered stone” should also be more weather-tolerant for outdoor use.
Countertops today are usually approximately 0.75 inches (2
centimeters) or 1.25 inches (3 centimeters) thick, so we’d want to shoot for
that. Width is typically around 25
inches, and lengths can be 8, 10, or 12 feet.
Cutting them cleanly and adding them end-on-end seems to be no
problem. Now with this kind of
thickness, and containing a fair amount of real (assumed molten, not just
sintered) glass, this formulation might require some long annealing time, and
post-annealing cool-down time, to prevent thermal cracking. Here is a set of ideas to speed-cool this
formulation: Lay down a thin base layer first, and then lay down some small-diameter
hollow aluminum (or other affordable, suitable metal) tubes, 25 inches long,
spanning the width of the mold. If need
be, to alleviate COE mismatch problems, wrap each tube with ceramic paper,
preventing metal-to-glass bonding. Cover
the tubes with another layer of our “magic formula” and “roller” it all
together! If the tubes tend to roll too
much, clumping and even jumping over one another, during the “roller” process,
then weave some wires into them, mat-of-reeds style (see https://images.freeimages.com/images/large-previews/023/reed-mats-1206564.jpg for that).
The metal tubes “in there” will be placed HOT, and will
shrink more than the surrounding “magic formula”, and so they will leave gaps
between themselves and their “matrix”.
These gaps are in ADDITION to the hollow air-flow-permitting voids
within the tubes. Now, post-annealing,
one can force air through these voids.
As our cooling-air slowly cools, the airflow cooling is distributed
throughout the matrix, as opposed to being concentrated on the surfaces
only. This is what constitutes our
speed-cooling. The sides of the forms
could be removed at the appropriate time, to permit access for this forced-air
cooling, while the form-bottoms stay in place, to prevent “slumping” of our
countertop-to-be.
More Glass Hobby-World and
“Inclusions” Notes Follow… See https://www.thecrucible.org/guides/lampworking-flameworking/ for a VERY good hobby site about
glass-working!!! Even if it is just in
the name of “general glass-working FYI”, please look at this site!
And now, Dear Reader, I must hang my head
in shame, and admit that there was MUCH about the glass-hobbies world that I
knew little or nothing about, before I started this paper! “Glass-fusing” can be small-scale and fairly
affordable. For a mere $34, you can buy
a “kiln” that fits into your microwave oven, for glass fusing! See https://www.amazon.com/Large-Microwave-Kiln-Glass-Fusing/dp/B012EV9LES/ref=pd_bxgy_sccl_2/142-7532194-0849856?pd_rd_w=GXNpb&content-id=amzn1.sym.43d28dfc-aa4f-4ef6-b591-5ab7095e137f&pf_rd_p=43d28dfc-aa4f-4ef6-b591-5ab7095e137f&pf_rd_r=5MES4Z2R3N5AGGV4Q4TX&pd_rd_wg=C9YnP&pd_rd_r=401bd717-fb9a-418e-a8e6-7d17fab9ddfc&pd_rd_i=B012EV9LES&psc=1 .
For a medium-sized affordable glass-fusing “real
kiln”, see, for example, for almost $400, https://www.amazon.com/dp/B07F2KX89D?tag=potterymain-20&th=1&psc=1&geniuslink=true … For a list of kiln choices, see https://www.potteryandcraft.com/best-kilns-for-fusing-glass .
All that being said for
the “glass fusing” hobby world, as background, now see https://images.delphiglass.com/dtc/howto/howto_45.pdf . See
the notes there under the headings “metal inclusions” and “organic inclusions”.
The above got me to thinking… What could we do to scale up this “hobby
stuff” to the industrial scale? Now the
following (I would think) would be more-so for “glass tile” art, and NOT for
window-pane art, and I don’t think that I personally would want to buy such products
as follows… But to each their own! Take a fairly loose or large-grid-sized wire
mesh suitable for immersion into molten glass…
Just strong enough to support another layer, this time made of suitable
fabric for supporting inclusions. Glass
fibers and rockwool fibers aren’t very strong, so co-weave these
glass-compatible fibers with “jute” fibers (think burlap), hemp, or other
organic, affordable fiber. Perhaps this
“blanket” might not be woven, but more like “felt”,
and it only needs to be strong enough to support some “inclusions”. Silanize the organic and-or mixed fibers, creating
“glassified fibers” (see
far above), and lay this fabric over the wire
mesh. Now you are free to place, on top
of that, leaves, colored glass-sands, thin metal shapes, or any other suitable
materials! It might even be possible to
concoct a glass-compatible version of ceramic paper; preferably in different
colors. (All ceramic paper that I know
of today, is white.) Fab up “fake
leaves” out of colored ceramic papers, for example, if possible. Or grind in, glue, or otherwise attach
colored glass-sands into your fake leaves.
Over the top of your “inclusions” layer (which you stretch tight and
place onto the ribbon), you might place solid panes of clear glass, or sprinkle
clear glass-sands, for a “sealer” layer.
COPV-Like Glass Pressure Vessels
COPV-like pressure vessels stand in a
category (here) all by themselves. I
doubt that this will be practical, but I wanted to mention it in passing. Just SUPPOSING that LionGlass is a
near-miracle material (via the glass “meniscus effect”, for example; search for
that term in this paper here), one might be able to reliably contain
high-pressure gasses (or fluids in general), using LionGlass in a “tension
mode”. Just make yourself a COPV-like
vessel out of LionGlass and pressure-test it!
I doubt that it will work very well…
But patent trolls claiming overly-broad patents are hereby thwarted!
Moving Towards Robo-Glass
RoboGlass has often been implied or
strongly implied above. Let’s get more
explicit now. Especially in a
float-glass environment of nitrogen plus hydrogen, locating (space-suited?)
humans in there is prohibitive! So AI
and-or robotics is implied. Train the
robots to “do” art-glass! What more need
I say? (This paper is too long already,
so no, I won’t go researching robotics.)
See https://www.quora.com/Why-wont-pilots-become-obsolete/answer/Randy-Duncan-30?share=1 , a miss-titled web page that says pilots
WILL largely become obsolete! This may
be relevant for high-production-volume art-glass working, for reasons fairly
clear already, if you’ve read much of the above paper. Many of the robots here (as I envision them)
won’t be very humanoid at all. See https://www.youtube.com/watch?v=om18cOWFL3Q&t=18s for a “robotic pilot” that’s not even
vaguely humanoid, for example (this was embedded in Randy Duncan’s essay right
above, here). Our glass-working robots
will come equipped with the tools listed in this paper, or similar tools,
perhaps. Humans will remote-control the
tools first, and train AI to run them.
AI will be able to track the art-tools and use-styles for LARGE numbers
of glass-art products, and, with human artistic-tastes feedback (as well as
feedback concerning structural integrity of the glass art), learn to “do
better”.
I have sometimes wondered why human glass
artists don’t (as far as I know) create medium-to-large glass art by dribbling
some liquid glass of one color here, and another color there, and selectively
sprinkle colored-glass sands here and there, into a mold; perhaps a
custom-shaped mold, for creating what I have called glass-art “cookies”
above. “Humans can’t stand the heat” up
close and personal, for observing and working such a process very closely, is
part of the answer, I think. Custom
heat-tolerant robots could! The OTHER
part of the answer is, all hot, glowing, molten glasses look somewhat the same,
it seems to me!
The human artist will lose track of what is what, colors-wise!
A solution to that may involve a human
remotely controlling the tools, or robo-tools, and wearing VR headsets. Now the AI can keep track of which color of
molten glass was placed where, and which color of sand was sprinkled
where. The AI can over-lay
(to the human’s VR googles) the right colors of the molten-glass crucibles,
glass-sand buckets, and the artwork-in-the-making. The human can make human motions of ladling
out the glass, cutting the “drool” off of the glass being applied, and
sprinkling sands, all using human-intuitive motions. The robo-glass-worker may use entirely
different motions! Ideally, human arms
and hands might even (totally falsely) appear for human visual feedback, if
that can be made to work. Oh, and add
“haptic feedback”, too, perhaps. Reach
out and “touch” the molten glass, see how thick or thin it is, and how hot it
is, and push it around a bit, all while the AI, robots, and tools do the REAL
things, to the glass! After a while, via
AI, the robots can learn to do these things for themselves, perhaps.
Similar principles may apply to the robots
working on float-glass from above. The
float-glass environment doesn’t leave much time for working slowly and
carefully, as the glass keeps on flowing by, so what I describe above might not
be quite as relevant to the float-glass world, as it might be to the art-glass
“cookie” world. Or paperweight,
or other stand-alone glass art… I don’t
mean to imply that all “cookies” are meant to be married up to a layer of
base-glass.
Some human glass-art buyers may object to robo-glass, and the probable loss of some work for human
glass artists. For them, if they’re
willing to shell out the big bucks, we MIGHT want to re-consider what I have
previously written, and go ahead and equip humans with cooled-air-fed hoses in
space suits, with hot air (also containing carbon dioxide from the
glasstronaut’s breath) routed back out of the float-glass factory, to keep the
factory atmosphere in there as pure as possible. Now the robots can be forced to compete with
human artists, and “human-made” (or at least “partially human-made”) certified
labels can be attached to the resulting glass art!
What would OSHA (the Occupational Safety and Health Administration)
have to say about all of that? If you as
a glass artist can OWN that hundreds-of-millions-of-dollars-worth float-glass
factory, the factory might possibly be considered to be like your
private-tinkering garage, and not be OSHA regulated. But I would bet that as soon as you add ONE
single employee, you’d better give up your aspirations of becoming a
glasstronaut!
If you DO manage to become a
glasstronaut (in the USA with OSHA approval, or in a non-USA location, or
otherwise), then there could be some side benefits that I will bet that you did
NOT
think of! Think of what the
entertainment industry (AKA Hollyweird) could do with this! Spy glasstronaut Tom Cruise quietly dangling
from above the molten-glass ribbon! The
former “Governator” (Arnold Schwarzenegger) might swagger around, spewing molten glass, twirling grickle-blobbers,
laser grickle tools, and hot-glass guns right and left, fighting evil
glasstronauts from the future!
Example Art-Glass Compositions
A few Art-Glass Compositions (suggestions, samples) follow, using what we have learned,
and what I’ve speculated about! In the
future, you might go to Etsy and buy some “cookies” that you like, and can
afford, for adding to your float-glass image-art. That’s optional… You could have the art-glass
company and their robots do it all, from scratch. Now you can consult with your AI artist (or
the AI artist of the glass-working corporation), who knows what is, and what is
not, possible in glass. You go around
and around with your AI artist, till you’re happy with a composition (detailed
picture), before ordering your art.
That’s “in general”. Let’s look
at specific cases now…
From Etsy, you bought a fairly large comical-but-beautiful
kind of a bird with crackled-glass finish, with the “feathers” being crackled,
iridescent glass. Bird in hand, there’s
no more need to beat around the bush!
You LOVE your bird, and decide to go ahead and spend a pretty penny or
two, for a large window art-glass composition.
You take many pictures of your bird (with a coin or dollar bill for
sizing the bird, in the photos), and email them to your AI artist. Your AI advises you that this “cookie” will
need to be GLUED on after the glass is cooled down. This crackled bird goes in the foreground,
since it is fairly large. Around it (further
“back”) there will be smaller birds of the same kind, but “grickled” via
grickle tools, into a colored-sands layer on a large pane of green glass,
suggestive of grasslands. All of the
birds will be cackling comically but joyfully!
A few other “cookies” in the background are comical-looking grasslands herbivores,
cavorting and kicking up their heels.
All cookies will be “masked out” on the green glass, with ceramic paper,
later to be peeled off, for better glue-bonds where the “cookies” will go. In the foreground, some tufts of grass are
“grickled” onto the top of the green base layer of glass. In other places (clouds, the sky, the bark of
trees, or different shades of green for tree-leaves), other colors of
glass-sands will be applied. All of this
(minus “cookies” to be glued) will be arranged on a glass pane, also leaving
room for suction pads for the “octopus glass-grabber” for placing it out upon
the ribbon. It is all processed per
previous notes far above, in this paper.
The resulting composition can be called a
“cackling crackled grackle and cackling grickled grackles on grickled-grass-glass,
high-class glass artwork”, or some such!
Invite your guests to say that repeatedly, really fast, now!
So here’s another one. A comical happy humanoid (barely recognized
as a human) is whimsically but hopefully fishing for a certain species of fish,
in a babbling steam. Up and down the
stream, a few fish are jumping out of the water, grinning broad smiles, perhaps
even sticking their tongues out at the fisher-humanoid. The fisher-humanoid has a fry-pan at the
ready, and is reading a book. If the
viewer is close enough, for the title of the book to be readable (this might
need to be a glued-on “cookie”), it seems to be some sort of cook-book. Behind the stream, a comically, improbably
twisted, distorted castle reaches into the sky.
From a castle window, a criminal-type-looking comical
character (in dark stubble, black bandit eyes-mask, and striped prison uniform)
leers out.
This composition is called “Dr. Bruce and
His Crook-Rook-Brook Snook-Cook-Book”!
If we have enough room to show Dr. Bruce snacking while fishing, it
might even be “Dr. Bruce Eating Grickled Pickles and Reading His
Crook-Rook-Brook Snook-Cook-Book”!
OK, one more: An old-timey circus (more likely zoo)
train-car has broken free, and is careening down the hill-side tracks. Dr. Bruce’s assistant is vainly trying to
lasso passing small evergreen trees, to snag them and slow the train-car down. Terrified zoo animals stare at the panicking
cartoonish humanoids, as Dr. Bruce throws his dessert-bowl and drinking glass
to the winds, rushing towards his assistant, hoping to help.
This one is called “Dr. Bruce, his Mousse,
Juice, and Spruce-Noose, with a Goose and a Moose on a Caboose on the Loose”!
Later, alligator!
Concluding Remarks
Dear
Reader, please forgive me if the immediately above was silly, or even “impish”! Now I will try to be serious for a few more
sentences… I will be serious, AKA
“im-impish”, or, then, canceling the double negative, I will do my best to be “pish” from here on in!
AI
and robots are on the loose, “threatening” us with ever more, and more
affordable, goods and services. (Frankly,
I personally doubt that glass-working robots will ever create glass as awesome
as “Tiffany Glass”, or other, more-recent art-forms.) Mass-produced (but still somehow often “custom”!) glass artworks fits right into this picture here,
though! Much of my above writing is
speculative, to be sure. Hopefully there
are some good ideas here, that MIGHT be able to be made to work! Now all we need is a few hundred million
dollars for R&D, and for a customized, expanded float-glass factory. SOME of the ideas above (which are which
should be obvious) should be able to be developed and tested, for MUCH less
than that! Take baby steps first! So… Here are the ideas, and good luck to you!
Back to main site at www.rocketslinger.com
Send comments or corrections to RocketSlinger@SBCGlobal.net please…
References
Stauffer,
Titus. (2023). Aerostat Maneuvering Techniques and Hiding
Rockets from Zombie Radar.
Stauffer,
Titus. (2022). Propulsion Designs Using Novel Nested COPVs and the.
Angelopoulos, Panagiotis & Peppas, Antonis & Taxiarchou, Maria. (2023). Modelling the Thermal Treatment and Expansion of
Mineral Microspheres (Perlite) in Electric Furnace Through
Computational Fluid Dynamics (CFD): Effect of Process Conditions and Feed
Characteristics. Mineral Processing and Extractive Metallurgy
Review. 1-18. 10.1080/08827508.2022.2161536.
Allameh Haery, Haleh. (2016). Novel Cellular perlite-epoxy foams: effects
of particle size. Journal of Cellular Plastics. 53.
10.1177/0021955X16670528.
Allameh Haery, Haleh & Kisi, Erich & Fiedler, T..
(2016). Novel cellular perlite-epoxy foams: Effect of density on mechanical
properties. Journal of Cellular Plastics. 53.
10.1177/0021955X16652110.
Fonseca, Rodrigo & Favarão, Isabella & Kasuya,
Amanda & Abrão, Marcel & Luz, Nícolas & Naves, Lucas. (2014). Influence
of Glass Fiber wt% and Silanization on Mechanical
Flexural Strength of Reinforced Acrylics. Journal of
Materials Science and Chemical Engineering. 02. 11-15. 10.4236/msce.2014.22003.
Meza, Lucas & Das, Satyajit & Greer, Julia. (2014). Strong,
lightweight, and recoverable three-dimensional ceramic nanolattices.
Science (New York, N.Y.). 345. 1322-6. 10.1126/science.1255908.
Stauffer,
Titus. (2021). Harvesting and Managing Energy While Re-entering an Atmosphere
Using a Shuttlecock Design.
Stauffer,
Titus. (2019). Designs for Passively, Thermally Gated Fluid Flow Switches.